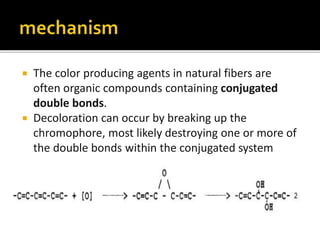
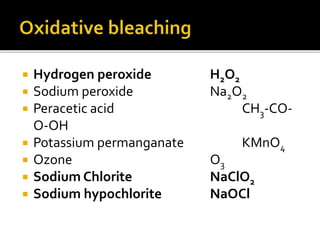
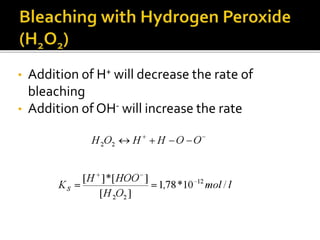
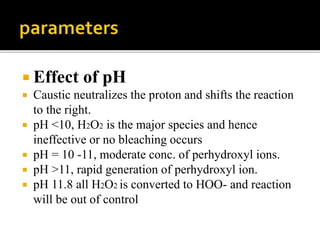
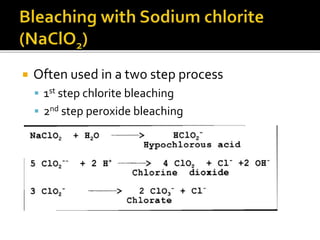
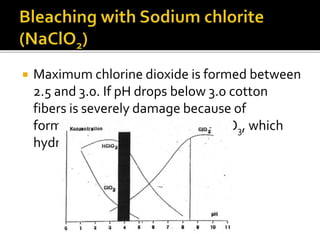
The document discusses various bleaching agents and methods. It explains that the aim of bleaching is to remove color from fibers through oxidative or reductive processes. Common bleaching agents mentioned include hydrogen peroxide, sodium hypochlorite, sodium chlorite, and chlorine dioxide. The effects of various parameters like pH, temperature, and metal ions are described for different bleaching systems. Sodium hypochlorite bleaching is noted to be the oldest industrial method while chlorine dioxide bleaching causes low fiber damage.