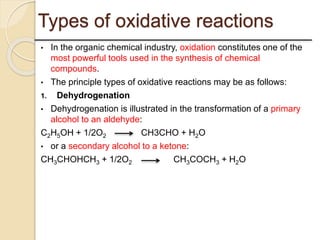
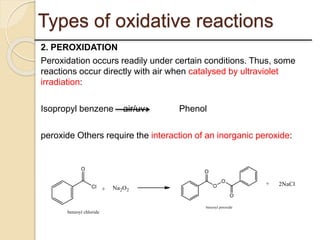
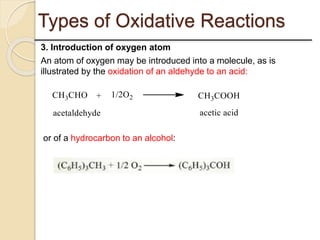
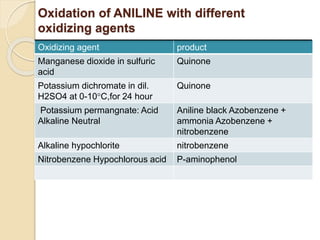
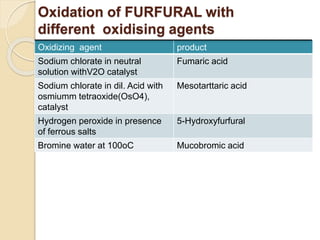
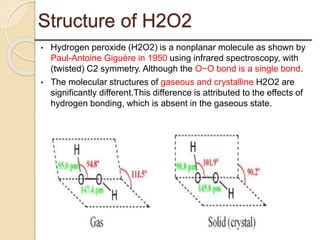
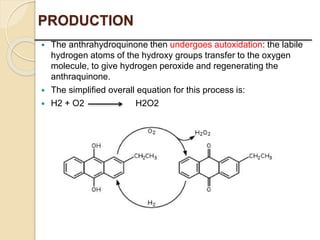
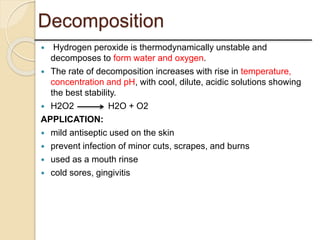
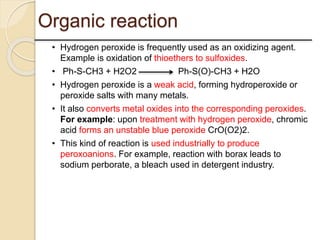
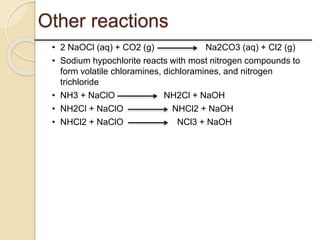
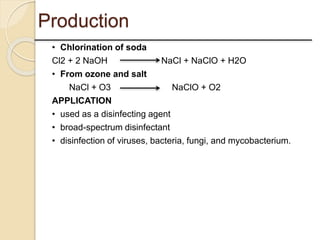
This presentation discusses oxidation reactions and various oxidizing agents. It defines oxidation as the gain of oxygen or loss of electrons by a molecule. The main types of oxidation discussed are dehydrogenation, peroxidation, and introduction of oxygen atoms. Liquid phase oxidation using oxidizing agents like hydrogen peroxide, sodium hypochlorite, and oxygen gas are also covered. Specific examples of oxidizing organic compounds like aniline and furfural with different agents are provided. Key oxidizing agents like hydrogen peroxide, sodium hypochlorite, and oxygen gas are described in more detail including their structures, production methods, decomposition reactions, and common applications.