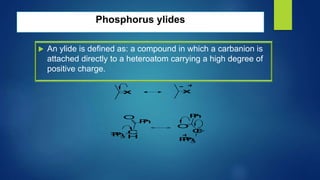
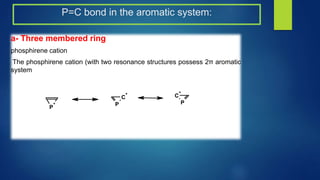
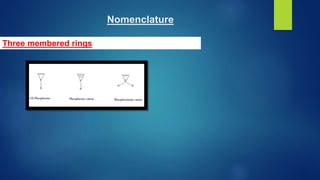
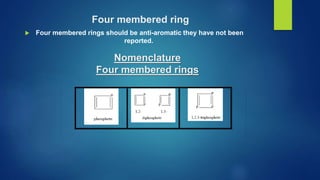
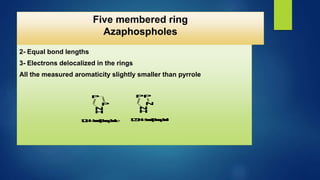
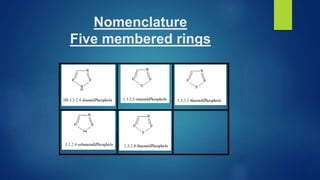
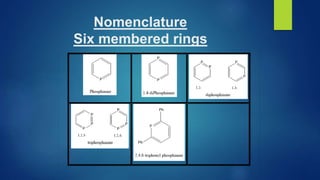
This document discusses phosphorus heterocycles. It begins by describing the preparation of phosphonium salts from phosphines and alkyl halides. It then discusses the reactions of phosphonium salts, including reactions with nucleophiles at the alpha-carbon or phosphorus atom that form phosphorus ylides. Phosphorus ylides are defined as compounds with a carbanion attached to a heteroatom with a positive charge. Methods for preparing phosphorus ylides from phosphonium salts or by addition to methylene ylides are also covered. The document analyzes the aromaticity of different sized phosphorus heterocycles from 3 to 6-membered rings, noting the planarity and bond lengths that indicate