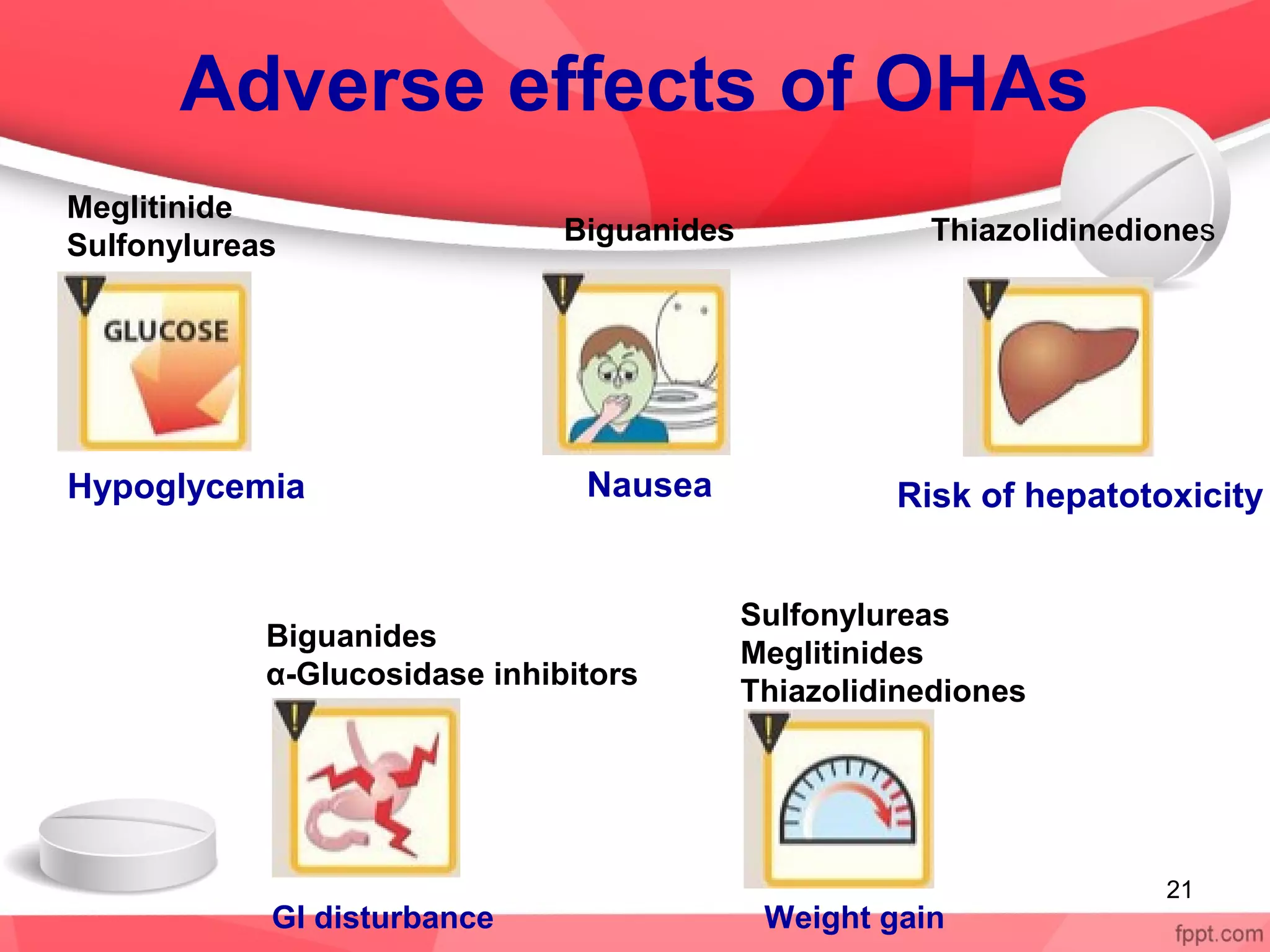
Oral hypoglycemic drugs are used to treat type 2 diabetes and work by lowering blood glucose levels. There are 5 classes of oral hypoglycemic drugs currently used: sulfonylureas, biguanides, meglitinides, thiazolidinediones, and alpha-glucosidase inhibitors. Sulfonylureas like glipizide stimulate insulin secretion from the pancreas. Biguanides like metformin reduce glucose production and absorption. Meglitinides and sulfonylureas both stimulate insulin secretion but meglitinides have a faster onset and shorter duration. Thiazolidinediones like pioglitazone increase insulin sensitivity. Alpha-glucosidase inhibitors