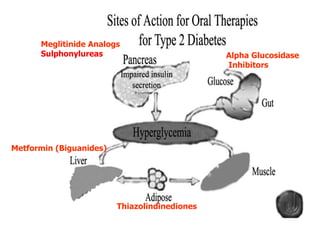
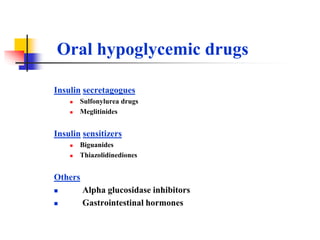
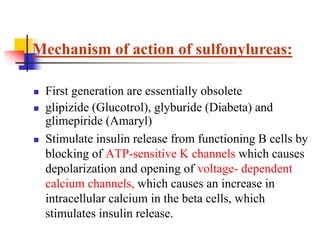
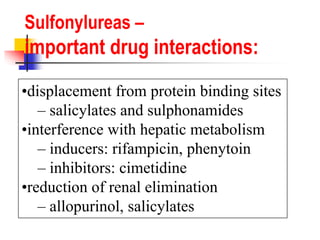
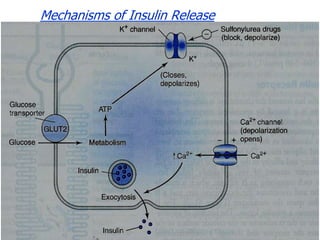
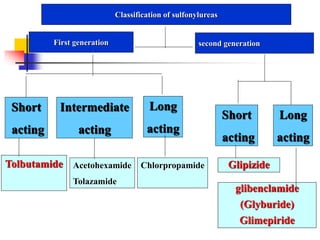
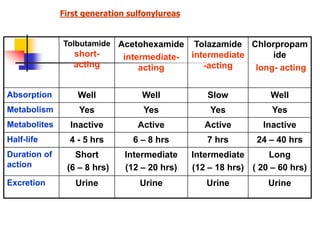
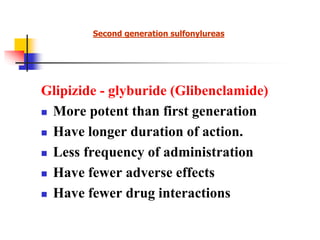
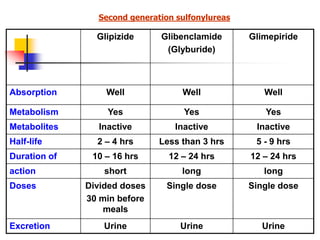
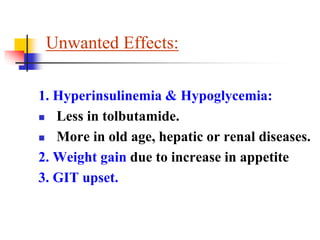
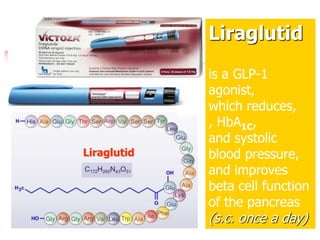
1. Oral hypoglycemic drugs are used to control diabetes mellitus and include insulin secretagogues like sulfonylureas and meglitinides as well as insulin sensitizers like biguanides and thiazolidinediones.
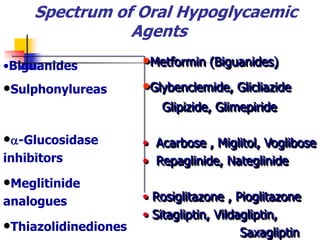
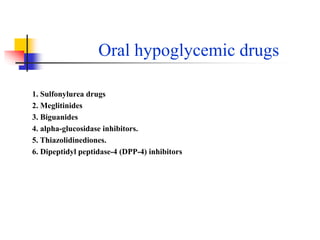
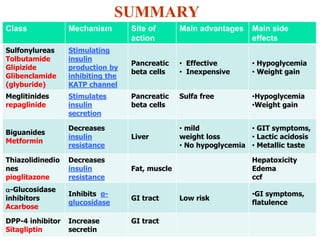
2. The main classes of oral hypoglycemic drugs are sulfonylureas, meglitinides, biguanides, alpha-glucosidase inhibitors, thiazolidinediones, and DPP-4 inhibitors with each having different mechanisms of action and side effect profiles.
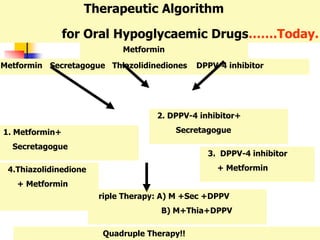
3. An ideal oral hypoglycemic agent would help conserve islet cell function to delay insulin use, have once daily dosing for improved compliance,