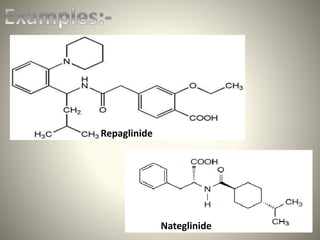
This document discusses different classes of antidiabetic drugs used to treat diabetes mellitus. It describes the classes as sulfonylureas, meglitinides, thiazolidinediones, biguanides, and alpha-glucosidase inhibitors. Sulfonylureas work by stimulating insulin secretion from the pancreas. Meglitinides also stimulate insulin secretion but have a faster and shorter acting effect than sulfonylureas. Thiazolidinediones improve insulin sensitivity. Biguanides like metformin reduce glucose production and absorption. Alpha-glucosidase inhibitors delay carbohydrate absorption in the gut. The document provides examples of drugs in each class and their mechanisms of action.