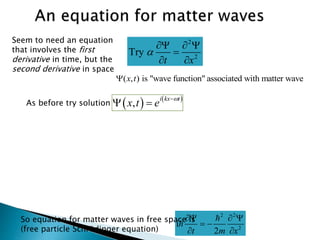
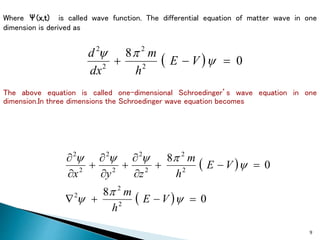
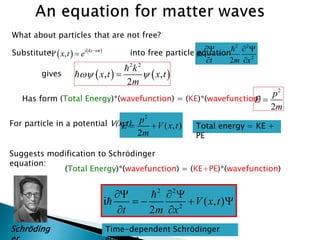
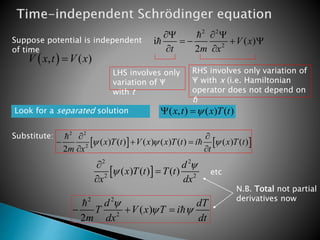
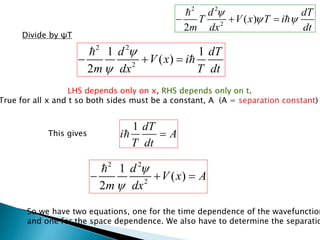
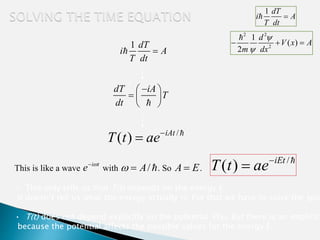
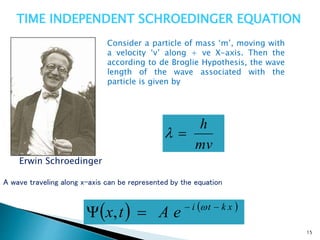
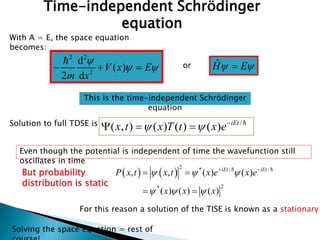
The document discusses the Schrödinger equation, which describes the wave-like behavior of matter and microscopic particles. It introduces the time-dependent and time-independent Schrödinger equations. The time-independent Schrödinger equation can be derived by separating the time and space dependencies of the wave function for situations where the potential is independent of time. Solving the time-independent Schrödinger equation provides the possible energy states of the system.