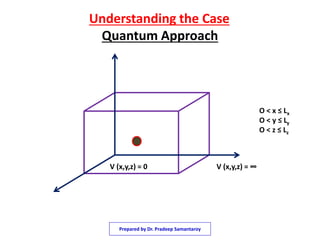
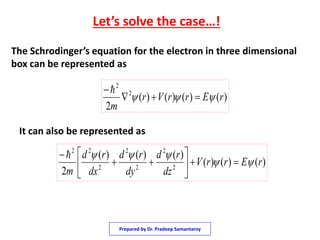
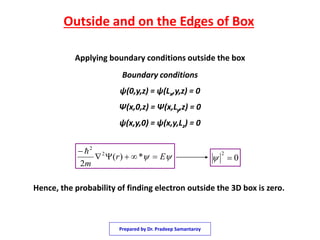
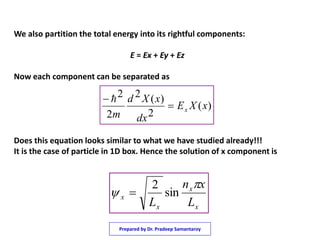
1) The document discusses solving the Schrodinger equation for an electron confined to a three dimensional box.
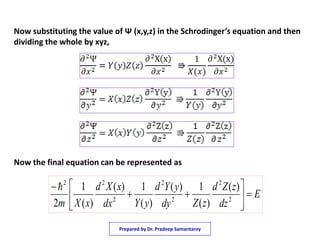
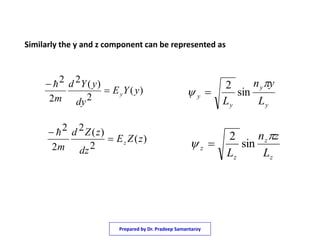
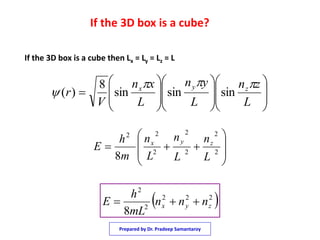
2) It shows that using separation of variables, the Schrodinger equation can be broken into three one dimensional equations that are each equivalent to the particle in a one dimensional box.
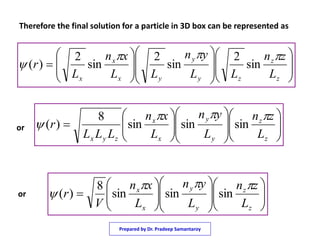
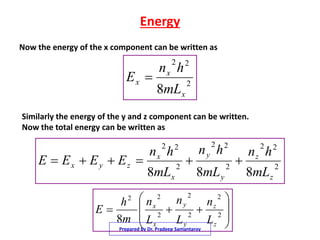
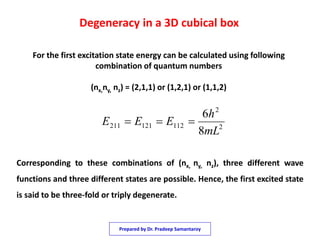
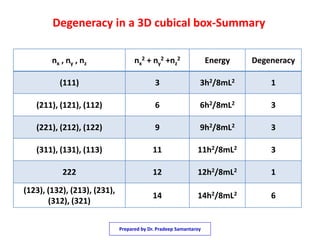
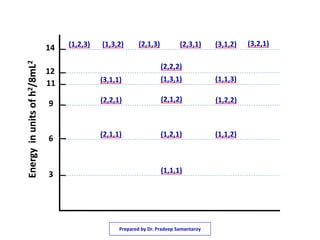
3) The solutions for the wavefunctions and energy levels are presented, with the energy levels increasing with higher quantum numbers and becoming degenerate with multiple combinations of the quantum numbers.