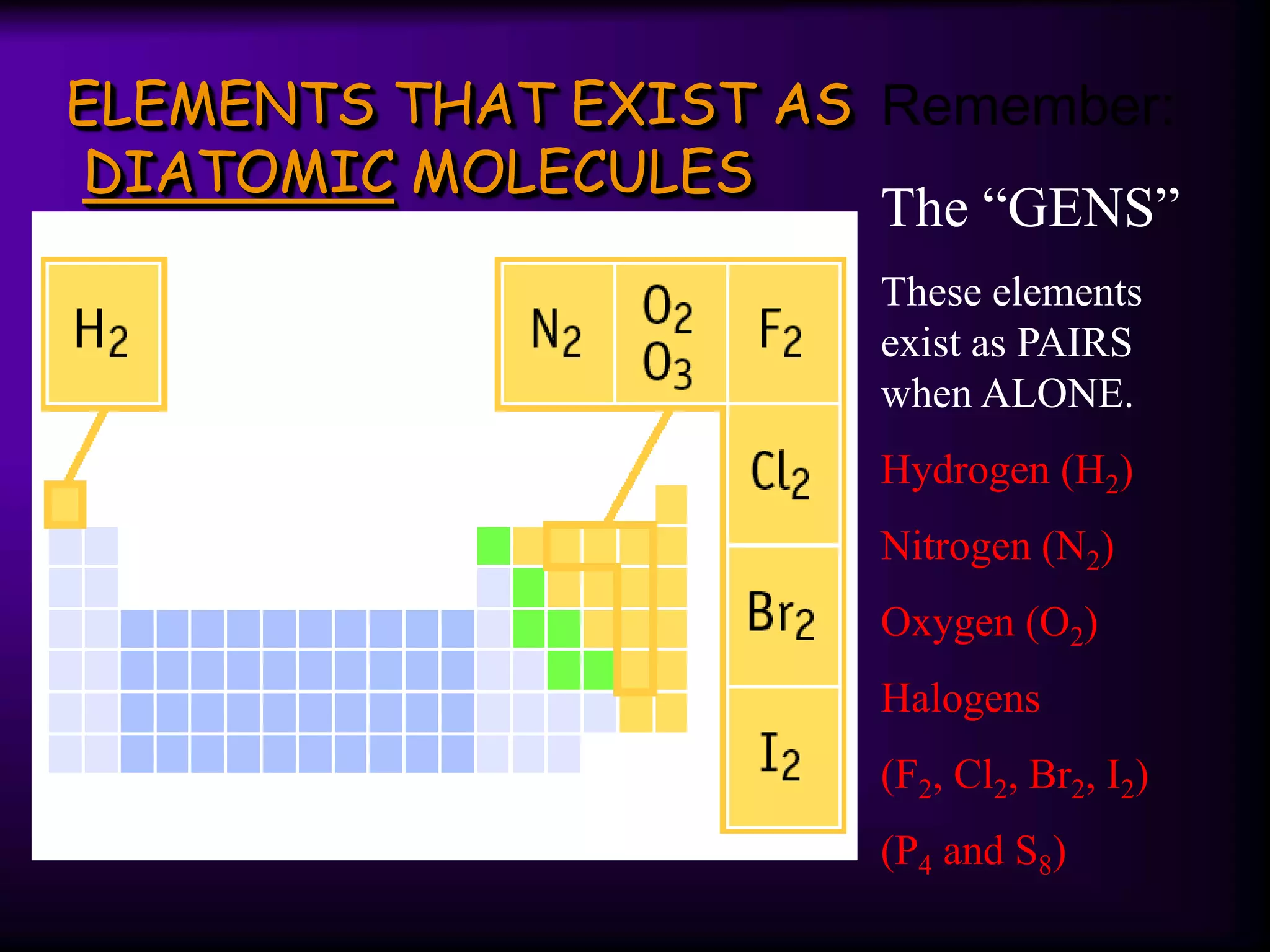
The document summarizes key concepts about the periodic table, including:
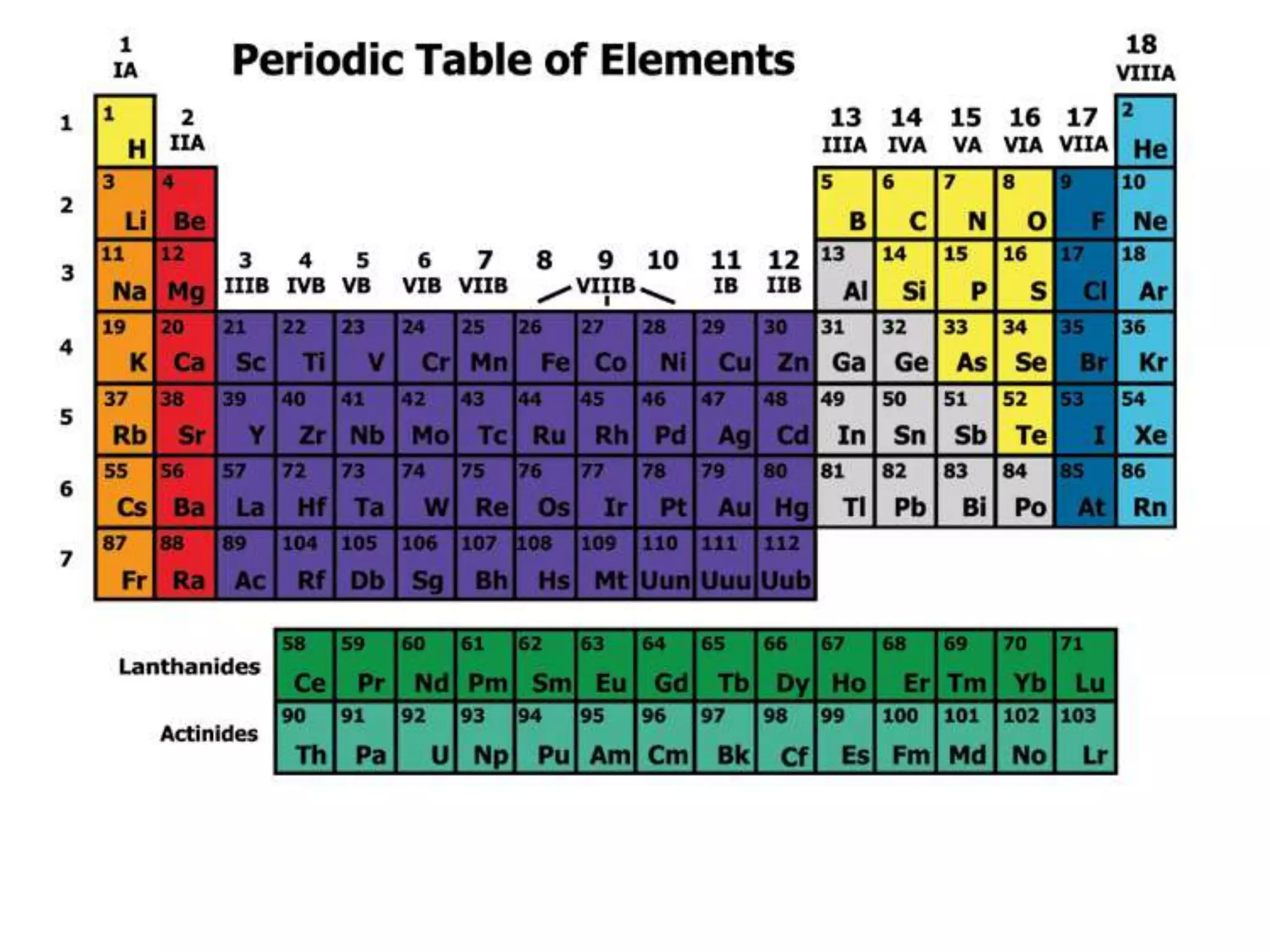
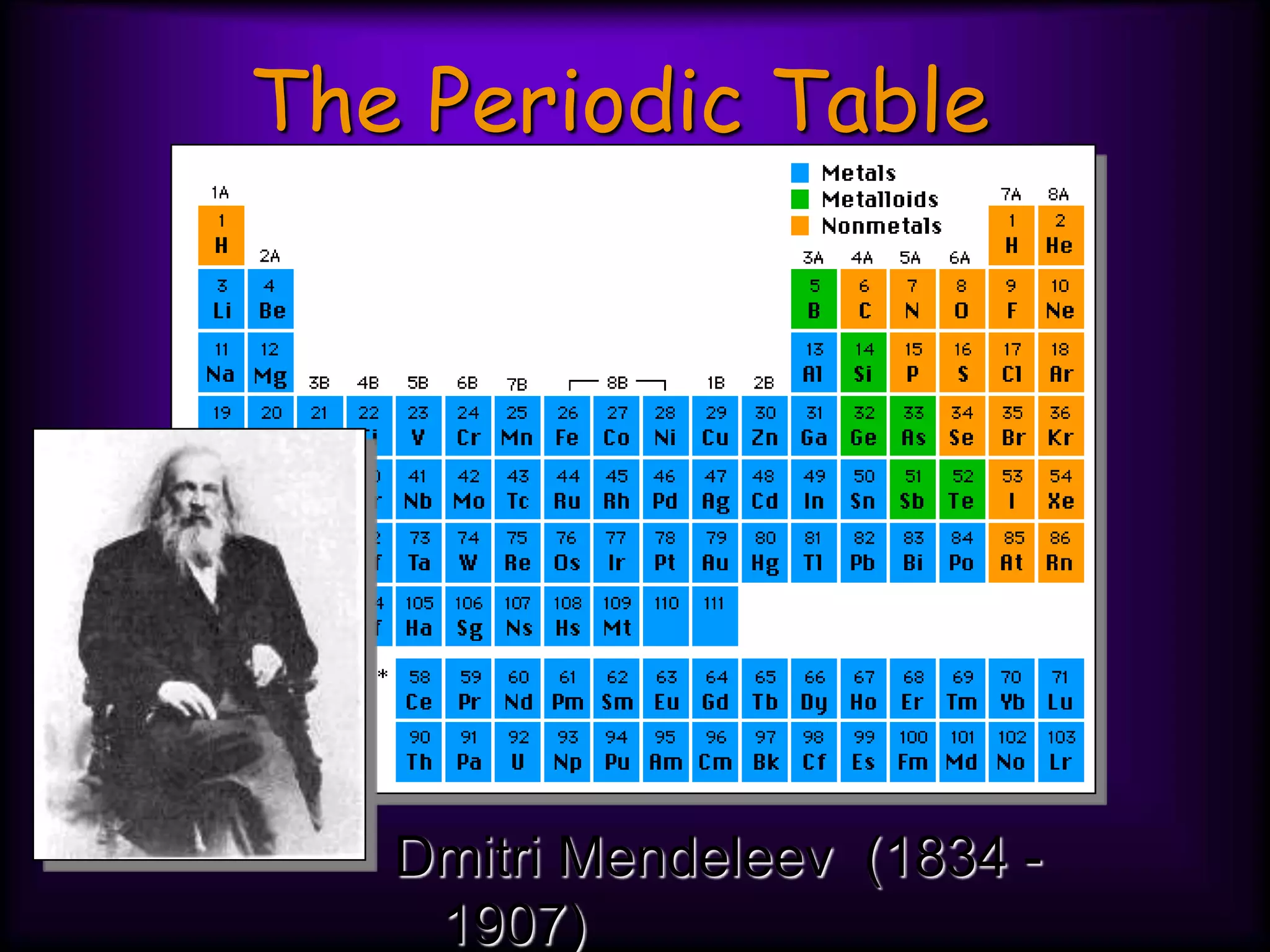
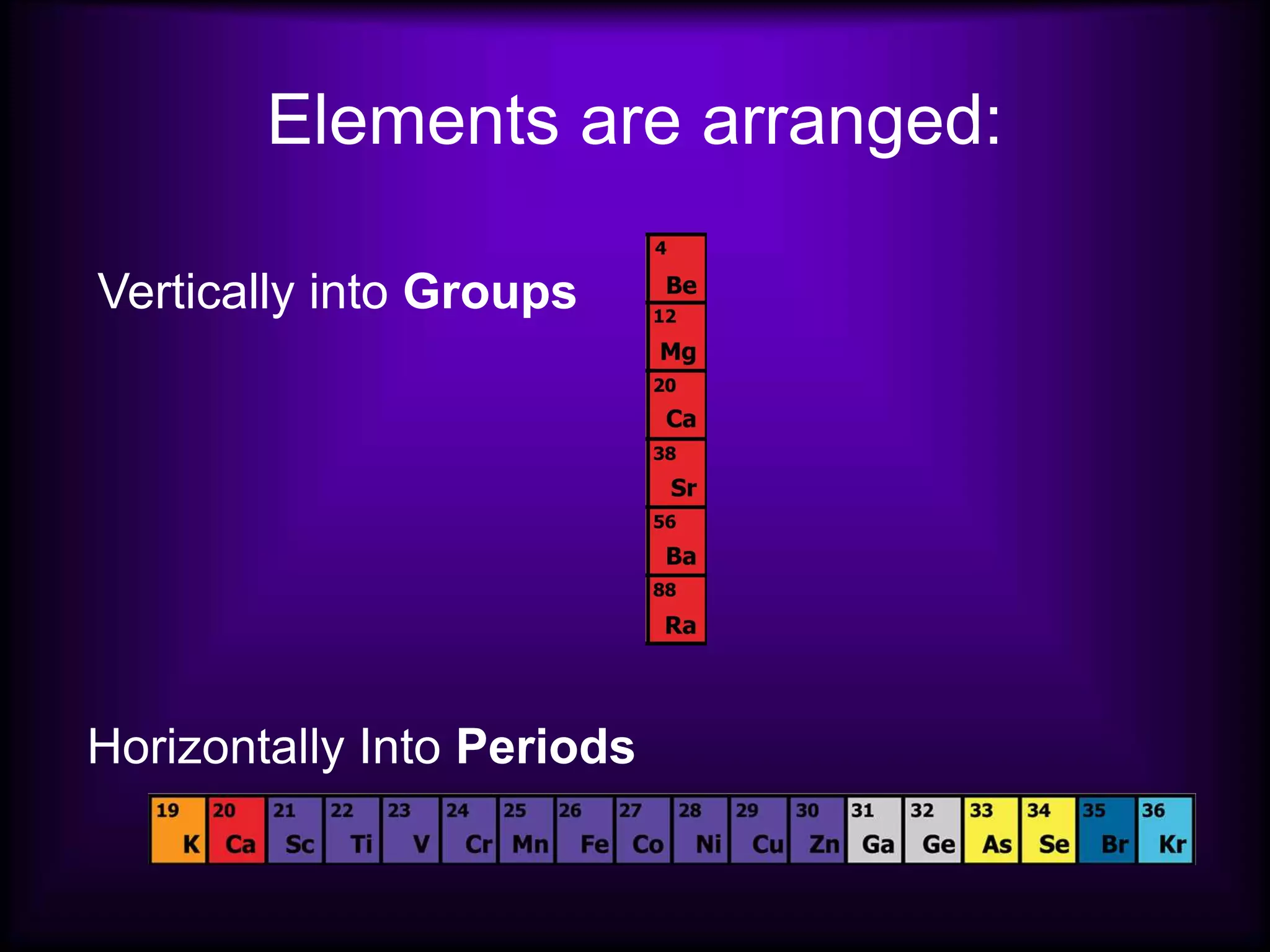
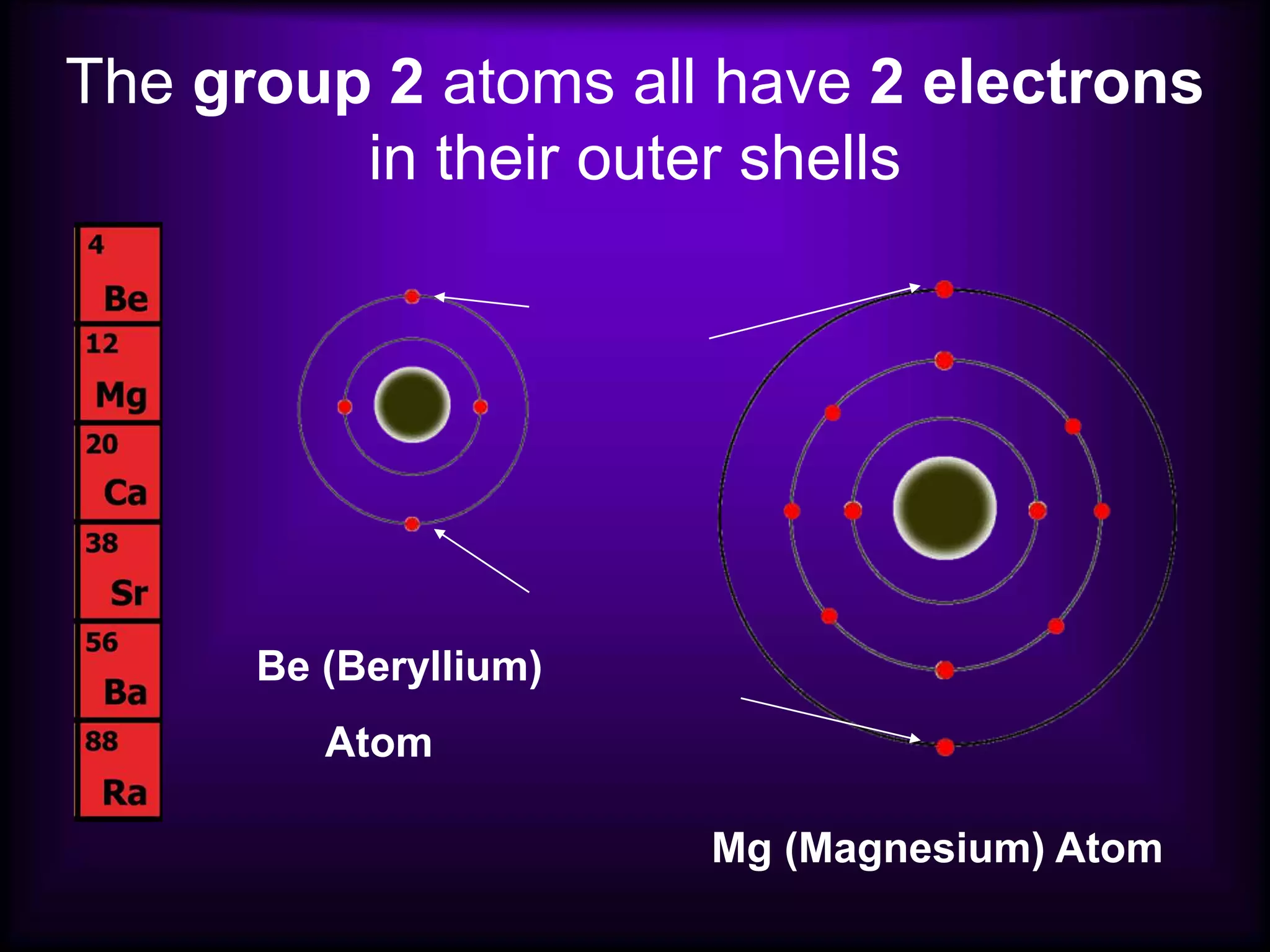
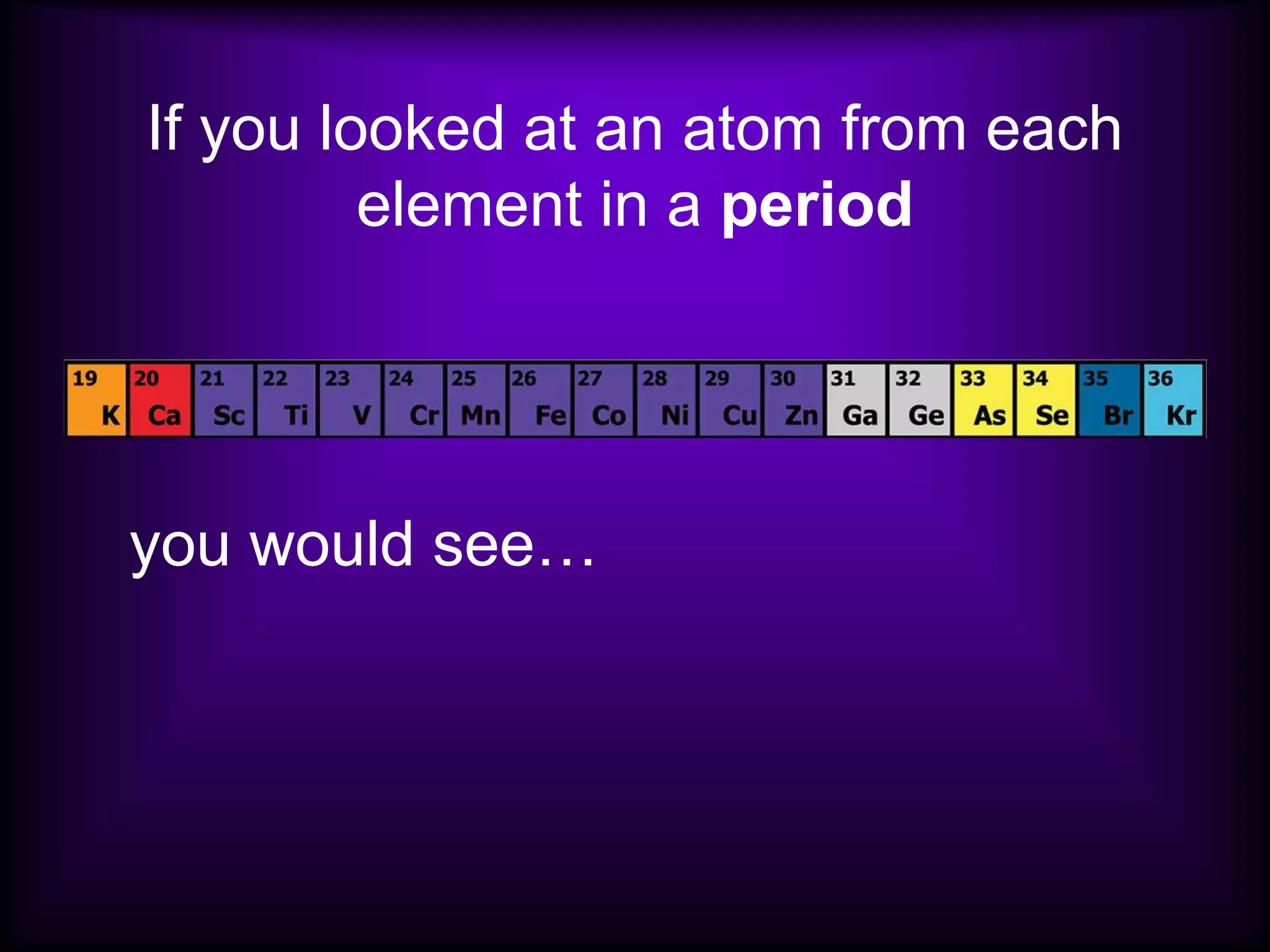
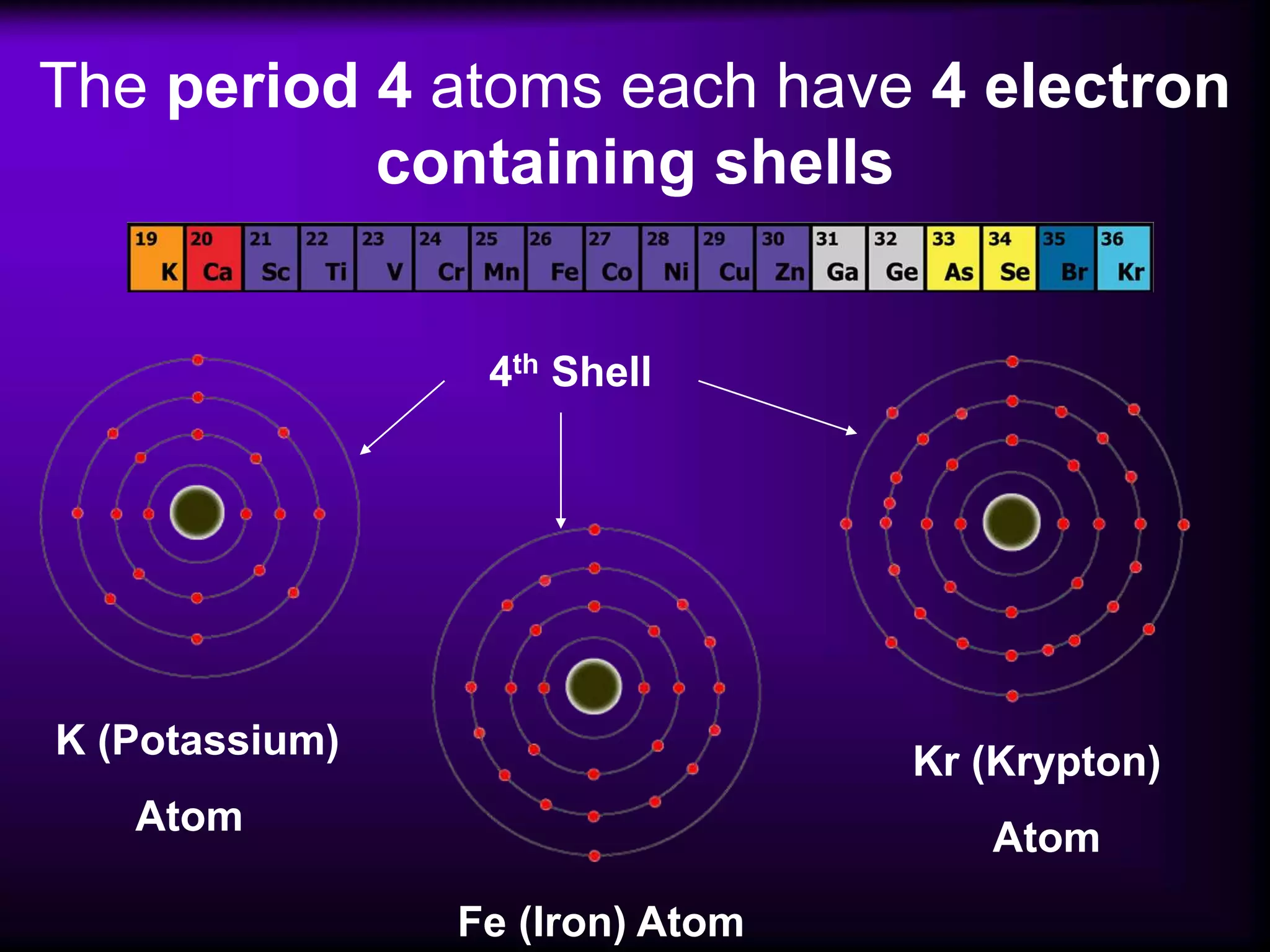
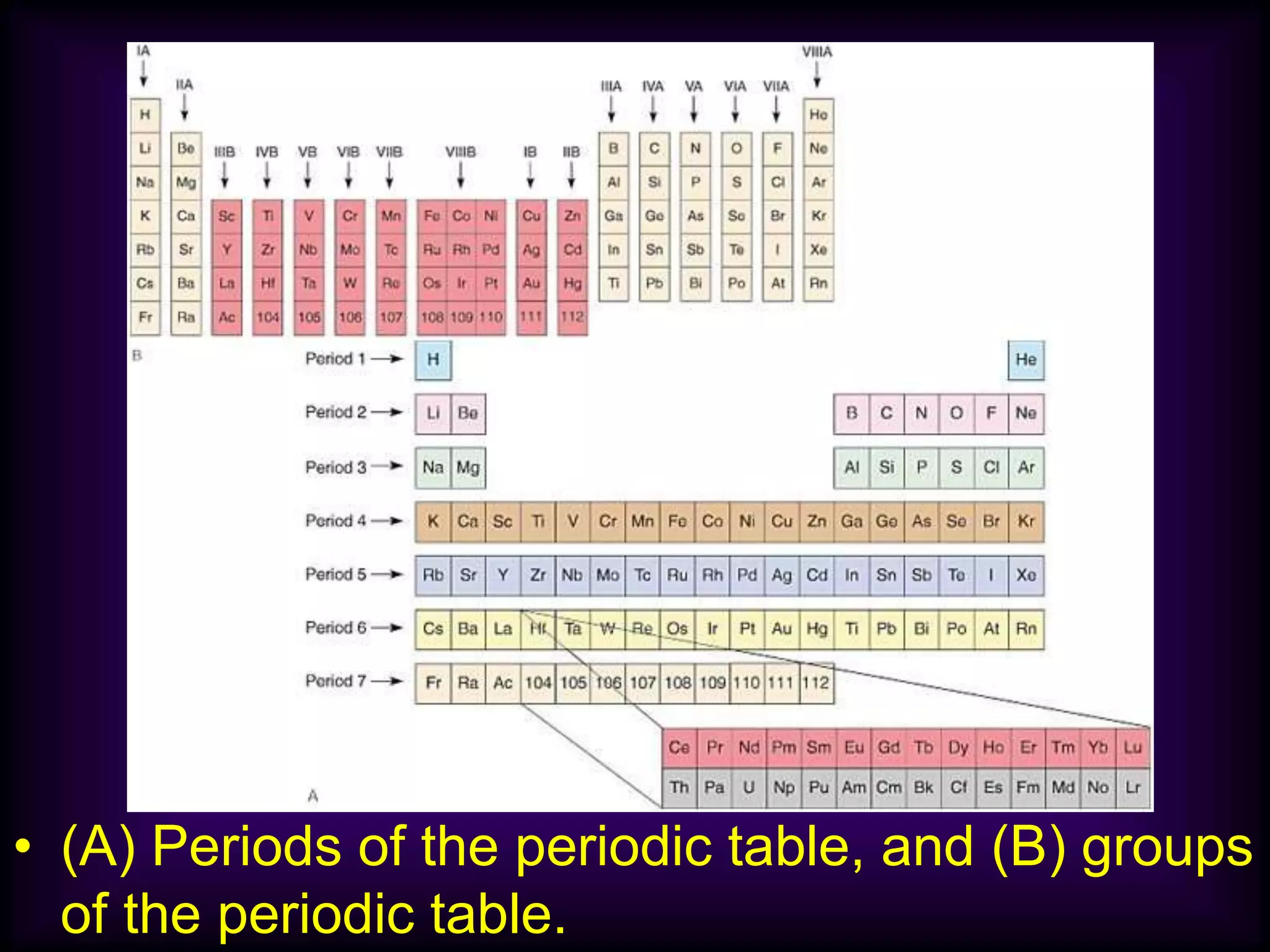
(1) Elements are arranged vertically in groups based on their outer electron configuration and horizontally in periods based on the number of electron shells.
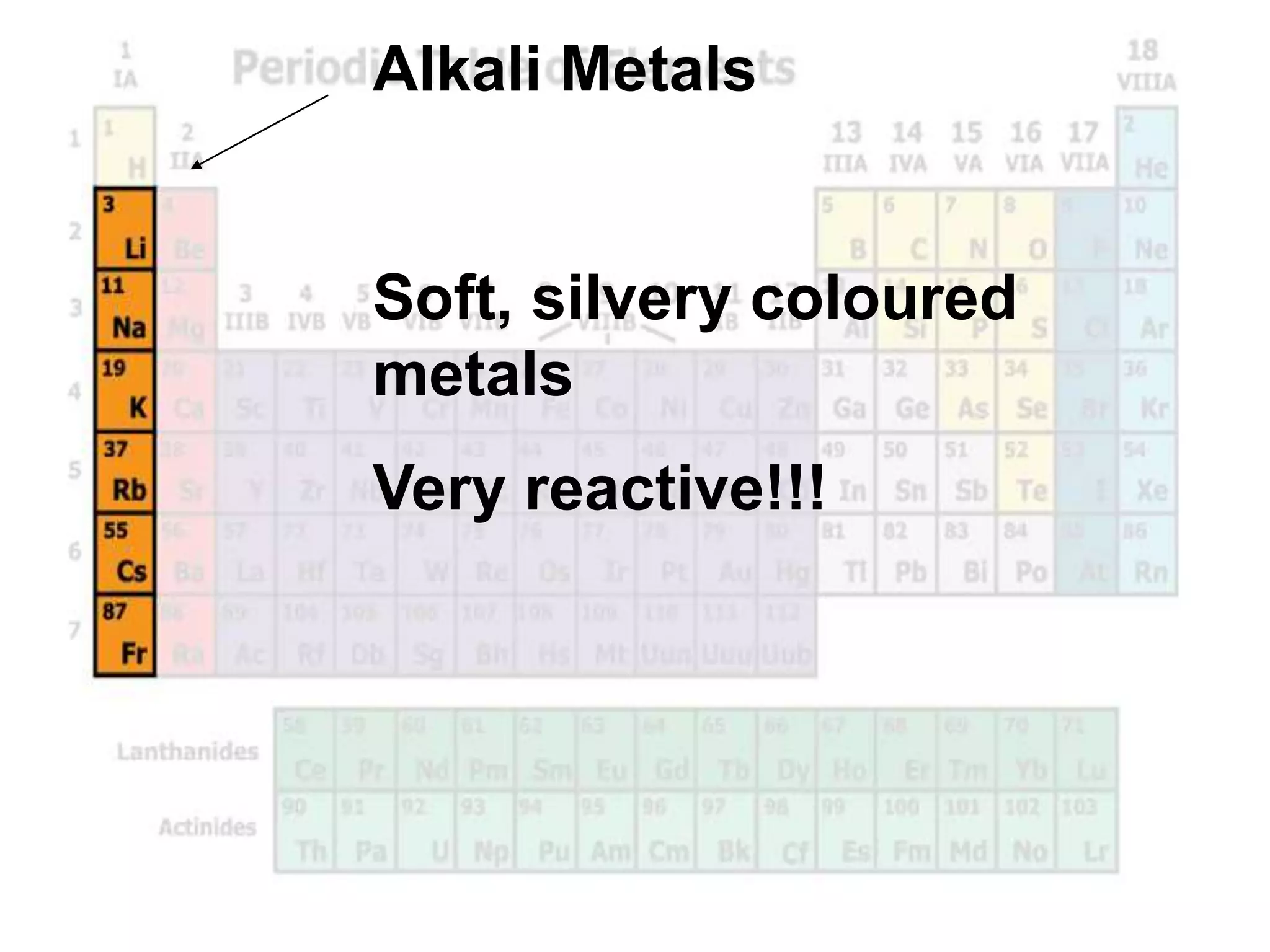
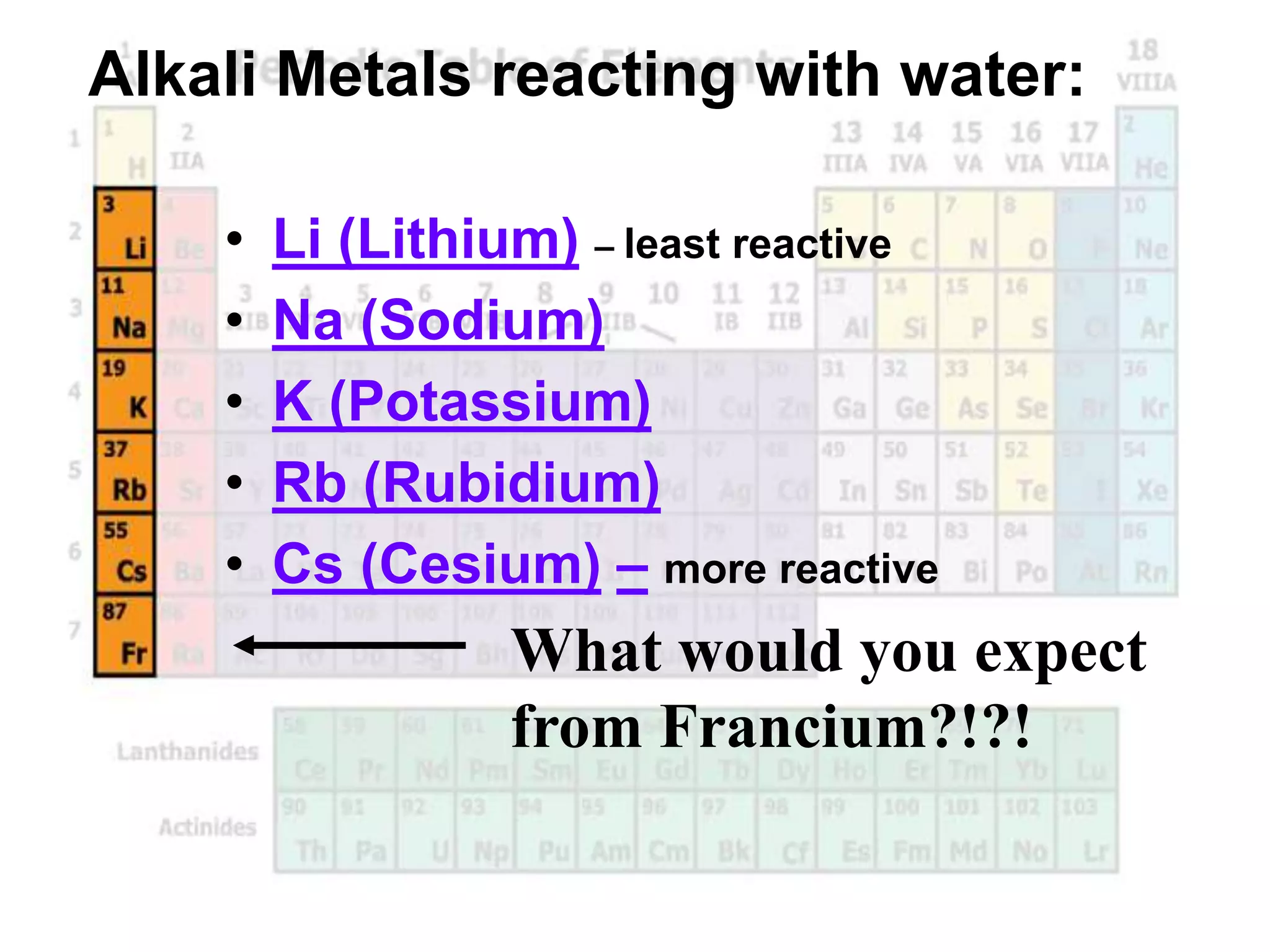
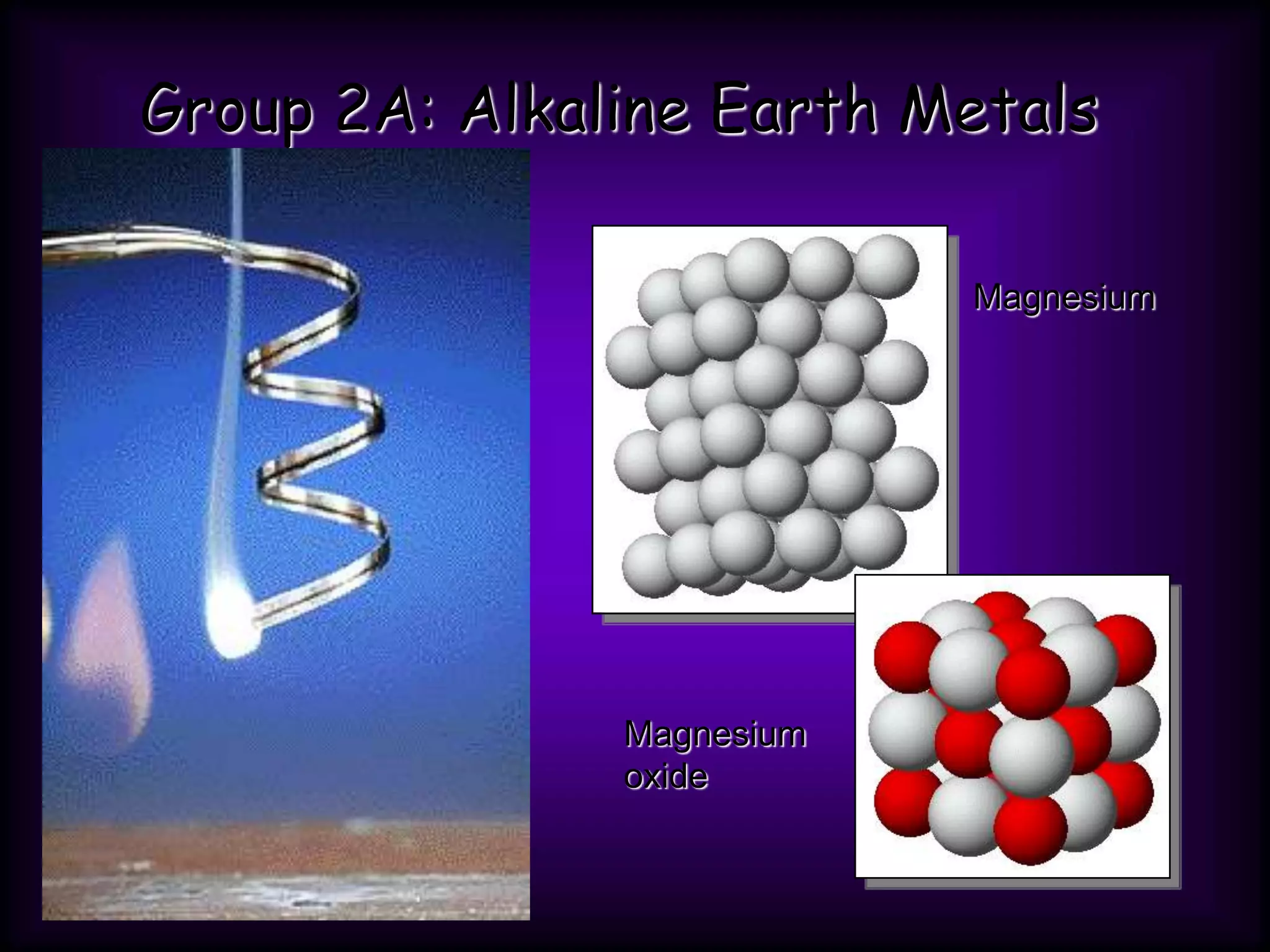
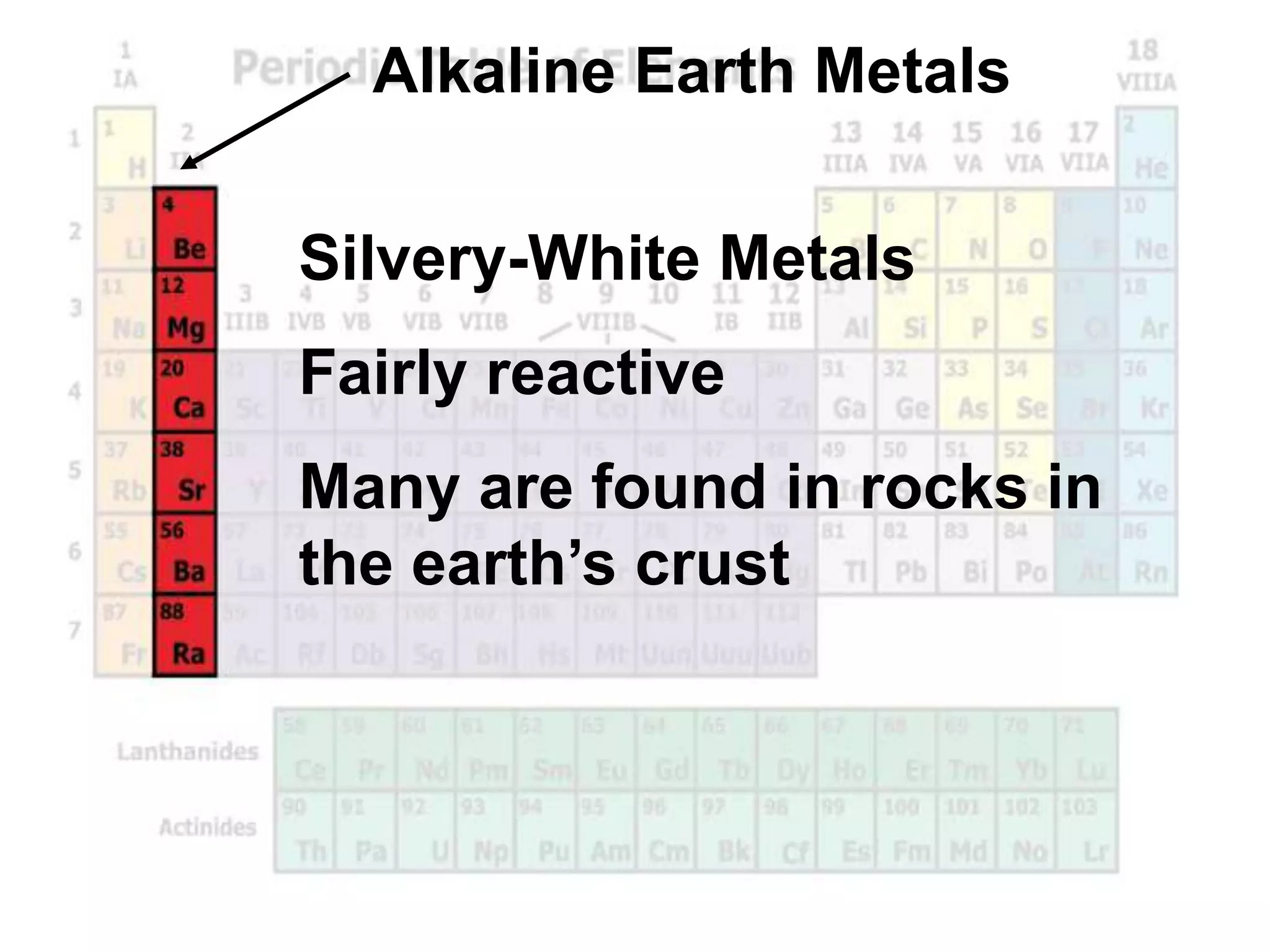
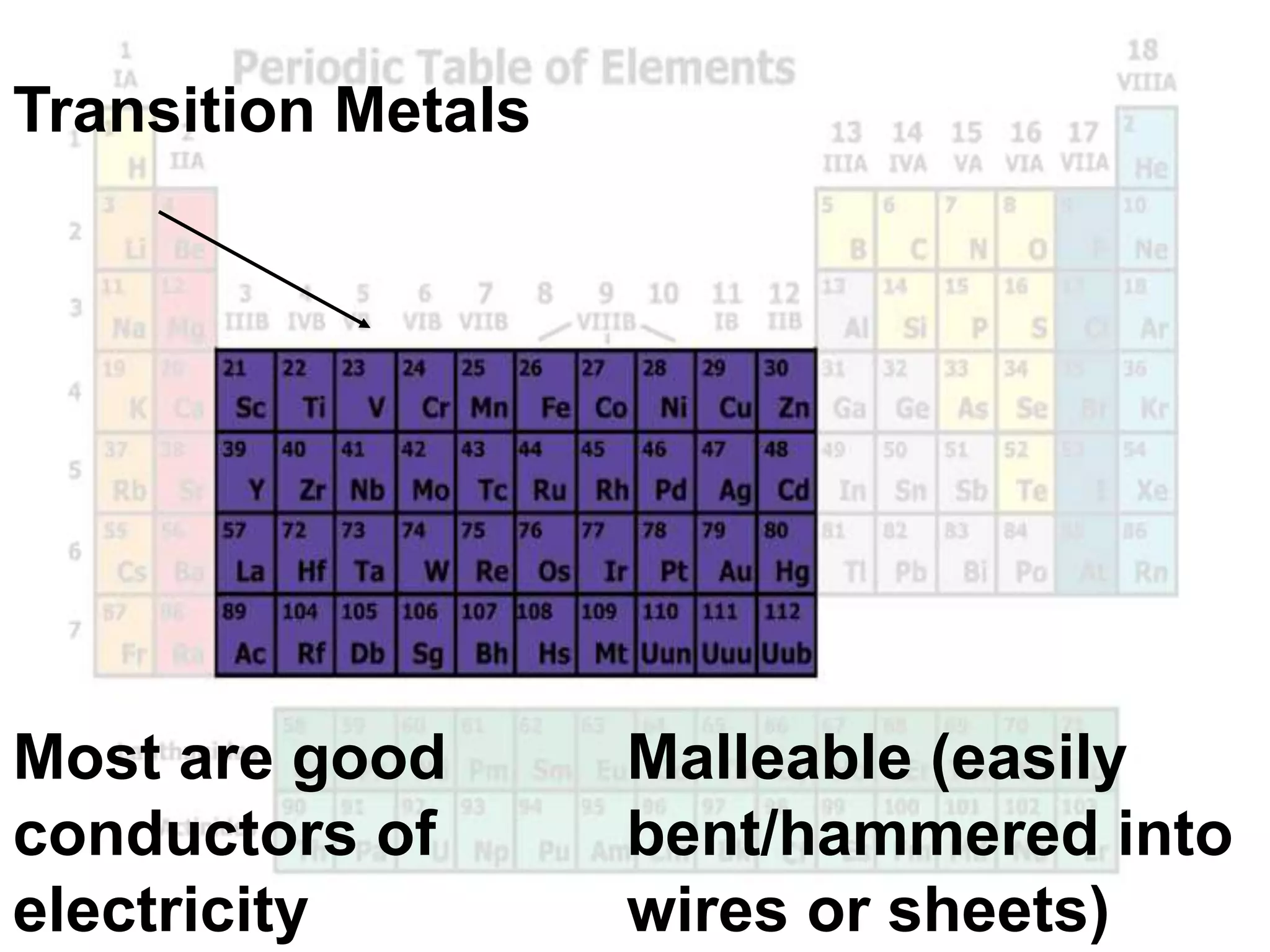
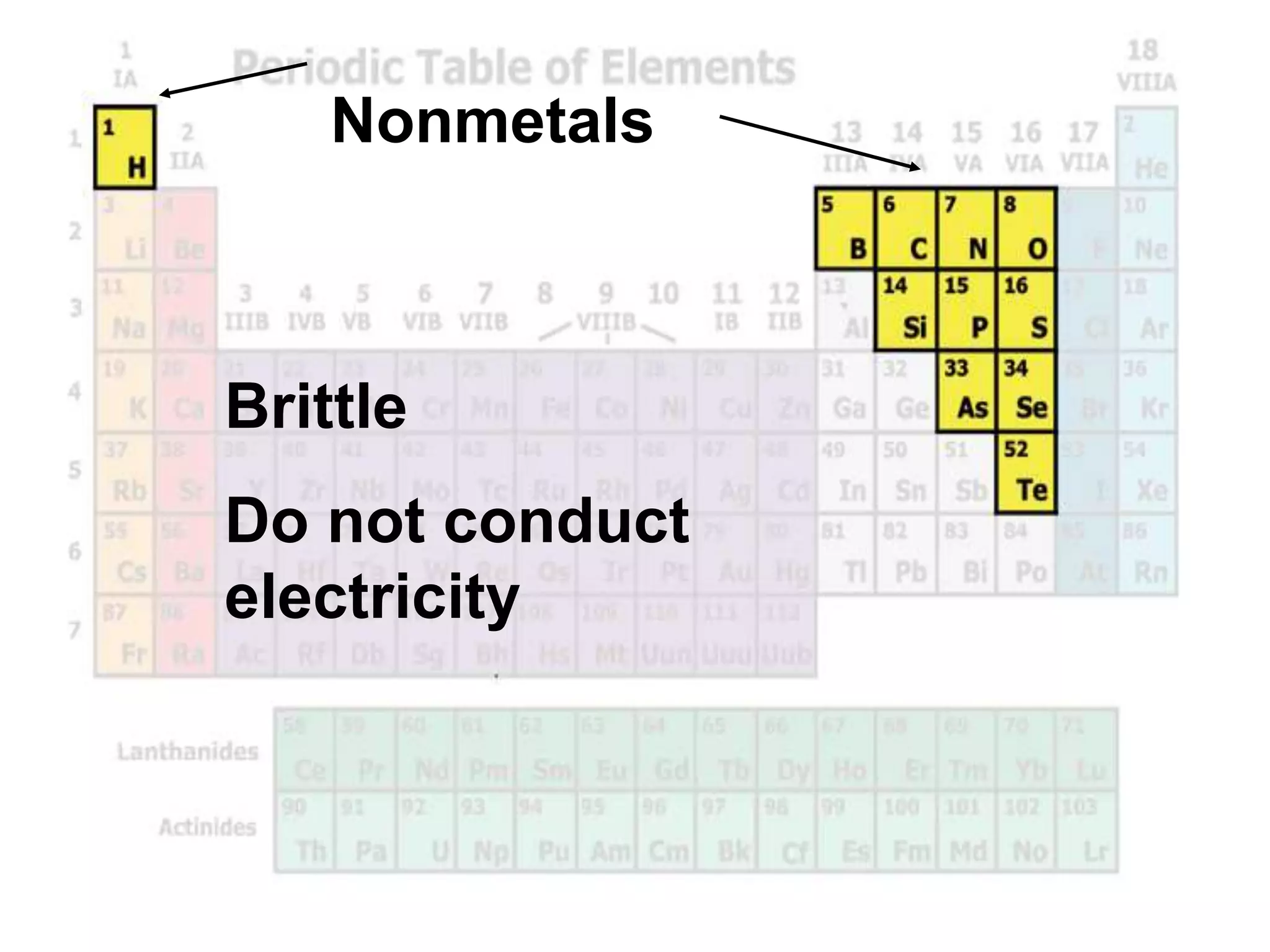
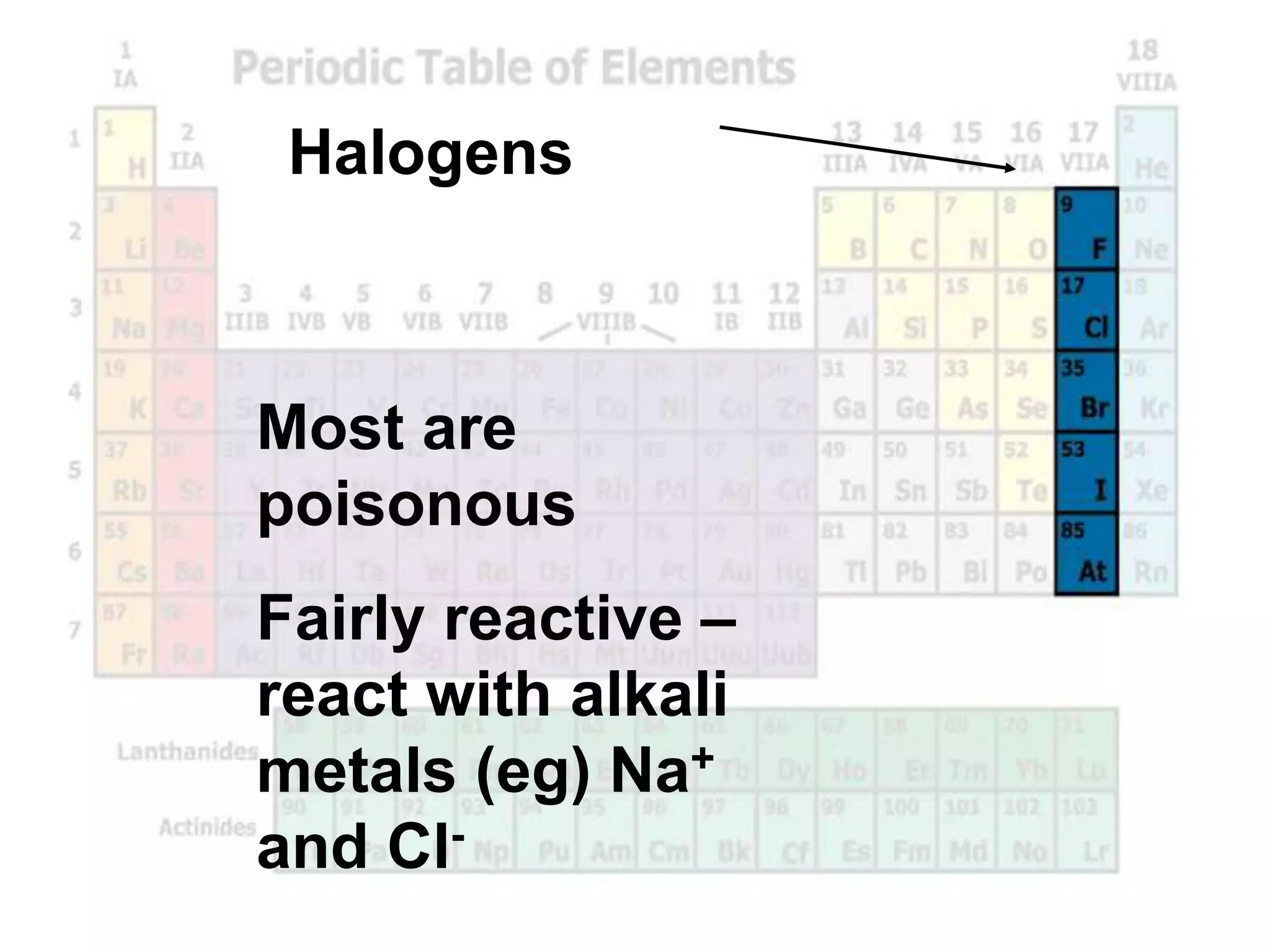
(2) Chemical groups such as the alkali metals (Group IA), alkaline earth metals (Group IIA), halogens (Group VII), and noble gases (Group VIIIA) have characteristic properties due to their outer electron configuration.
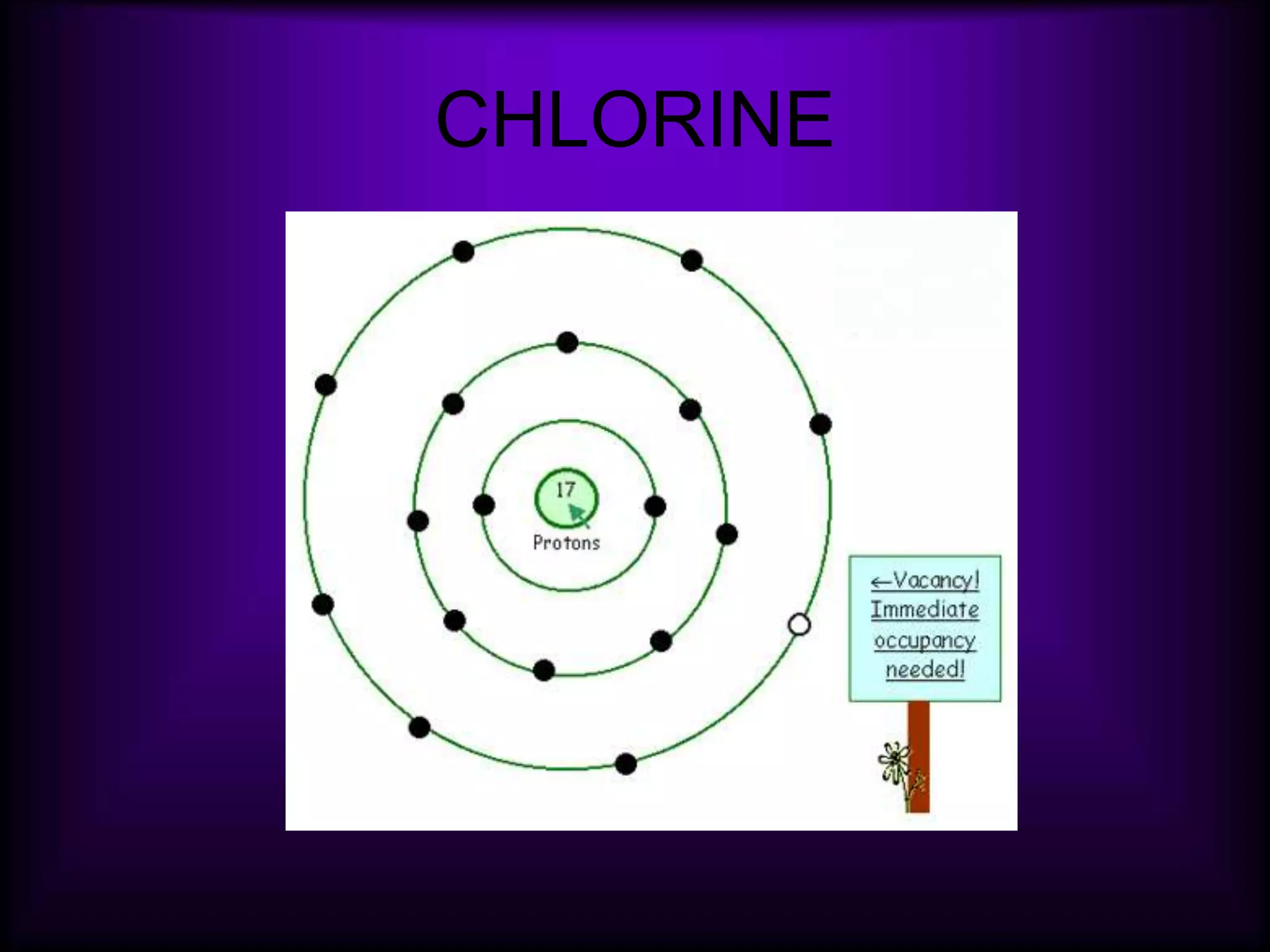
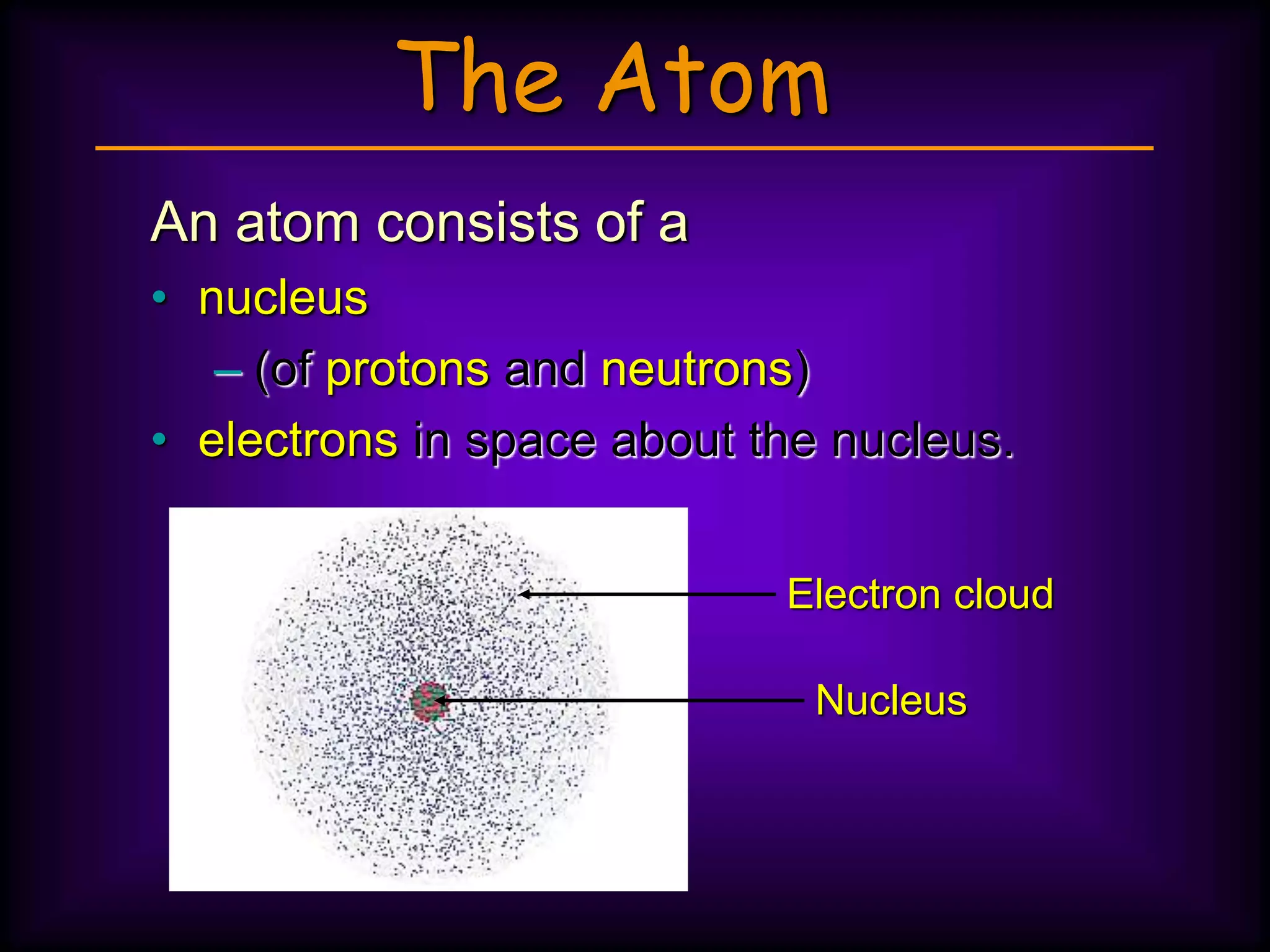
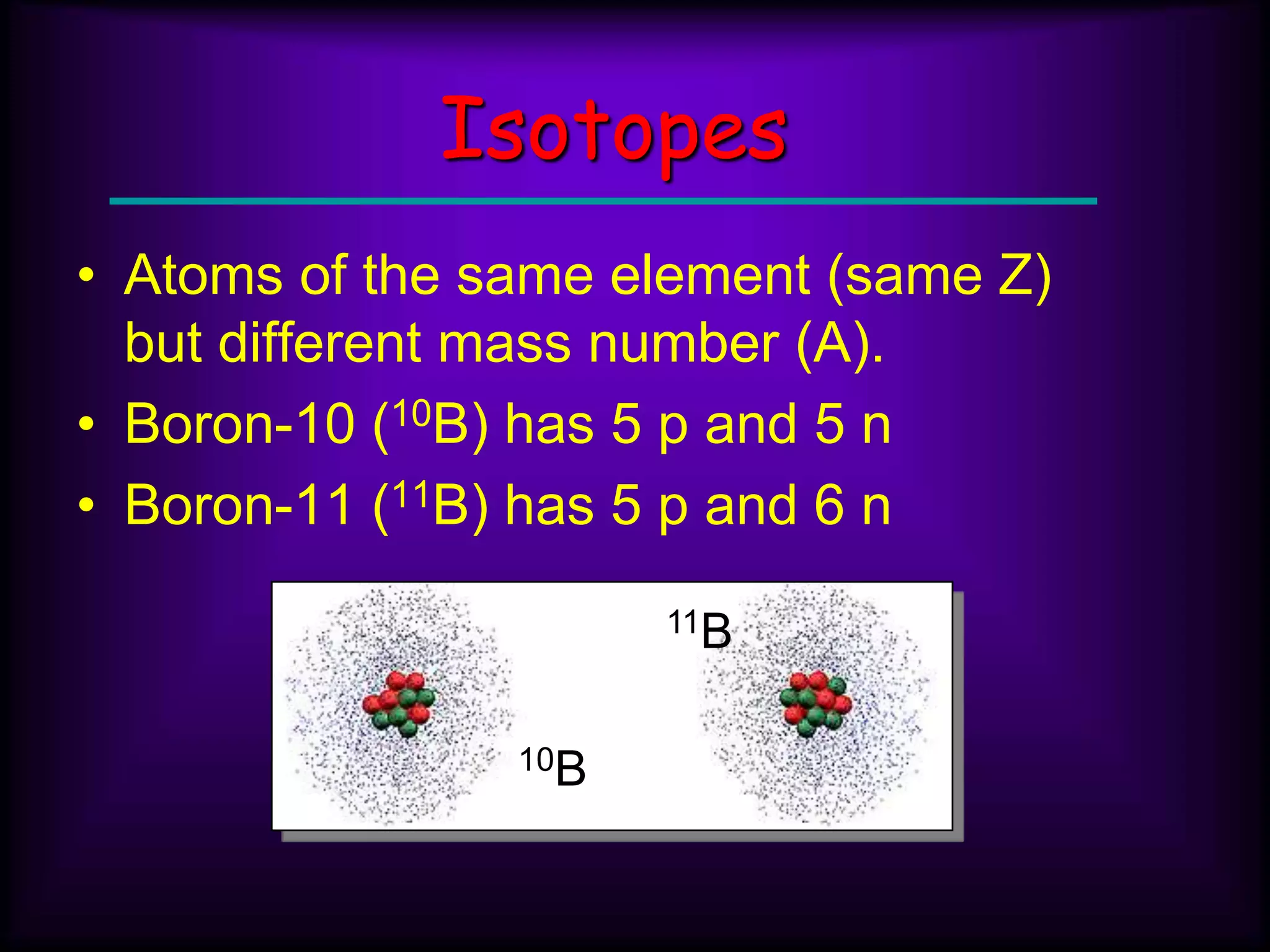
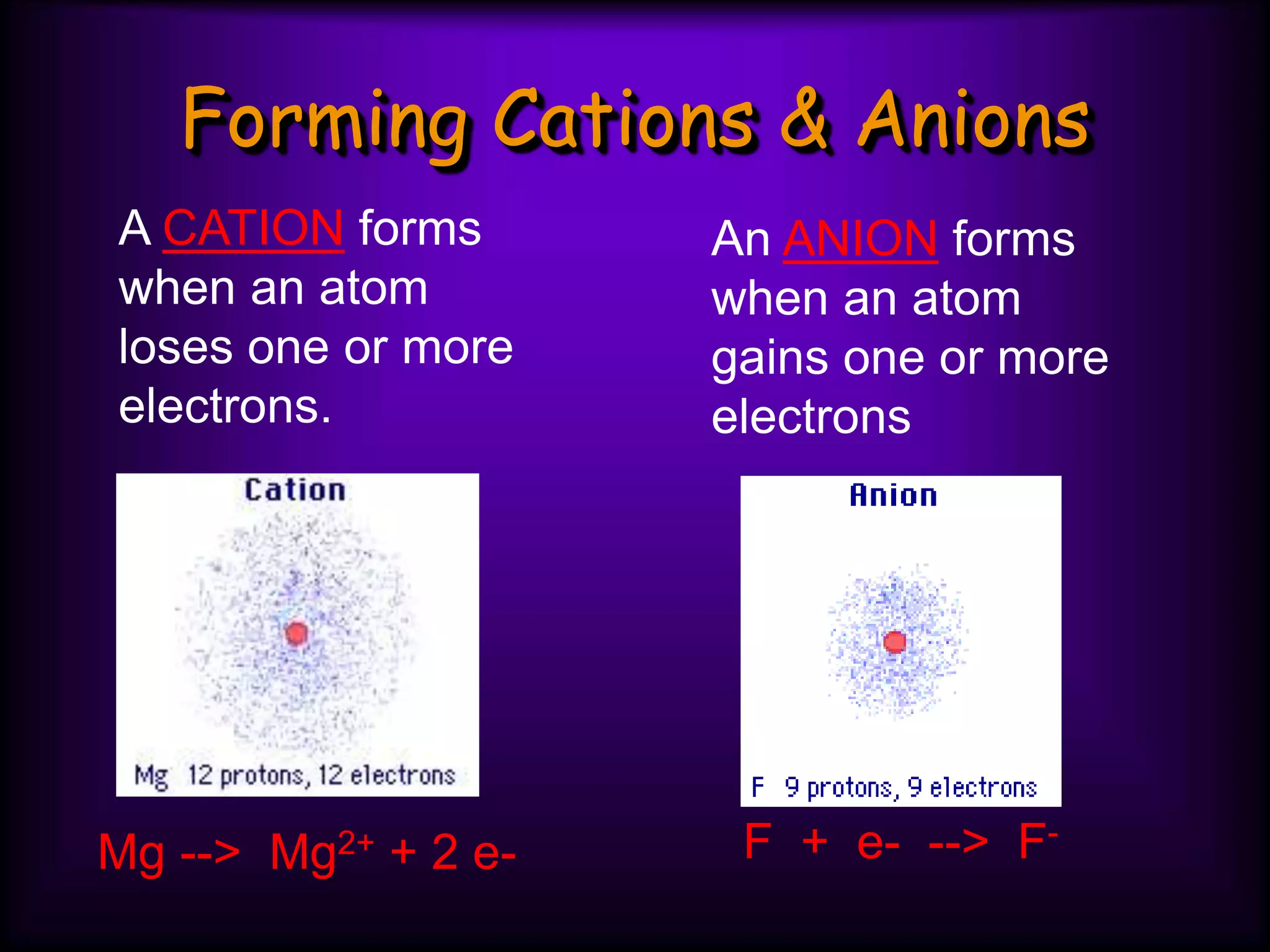
(3) Atoms consist of a nucleus of protons and neutrons surrounded by electrons arranged in shells. The number of protons determines the element. Isotopes are atoms of the same element with different numbers of neutrons.