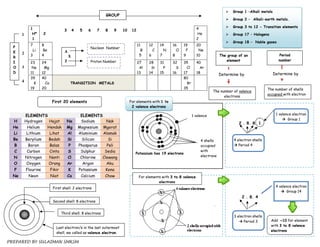
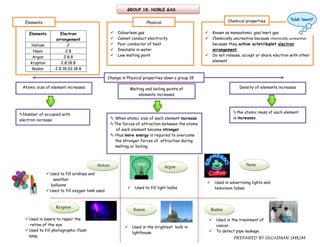
1. The document discusses the periodic table and provides details about various groups of elements. It describes the physical and chemical properties of Group 1 alkali metals, Group 17 halogens, transition elements, and Group 18 noble gases.
2. Key physical trends mentioned are that properties like melting point, boiling point, and density increase down a group as atomic size increases. Chemical reactivity also increases down a group as electropositivity increases.
3. The periodic table arranges elements based on proton number and electron configuration, allowing prediction of trends in properties across periods and down groups.

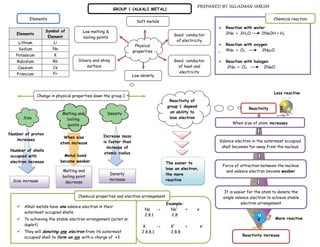


![Chemical reaction of Group 1(alkali metal)
Aim: To investigate the reactivity of alkali metal toward React with oxygen gas to produce metal oxides [white React with chlorine gas to produce metal chlorides
water solids]. [white solids].
PS : How does the reactivity of alkali metal toward water
HyPo: when going down a group 1, reactivity of A.M toward Metal G1 + O2 → metal oxide Metal G1 + Cl2 metal chloride
water increase
Gas jar spoon Gas jar spoon
Metal*
White fume Gas jar White fume
water White
Gas jar
Variable Action to be taken Metal* Metal*
Repeat exp using diff. alkali Oxygen gas
M Metal G1 PROCEDURE:
Chlorine gas
Chlorine
metal
Reactivity of Observe movement alkali PROCEDURE:
R
alkali metal metal on water surface PROCEDURE
Size of alkali Use same size of alkali The lithium is heated in jar spoon until its start
C burn and put into gas jar containing oxygen gas The lithium is heated in jar spoon until its start
metal metal
The observation is recorded burn and put into gas jar containing chlorine gas
When the reaction stops, 5 cm3 of distilled water The observation is recorded
RESULT: is poured into gas jar and solution formed is The experiment is repeated with sodium and
Metal* tested with red litmus paper potassium
Observation
Reaction becomes more vigorous
The experiment is repeated with sodium and
Lithium moves slowly on water surface potassium RESULT:
with ‘hiss’ sound. Metal
Reaction becomes more vigorous
Li The colourless solution formed; turns red Observations
RESULT:
litmus paper to blue.
Sodium moves quickly on water surface Metal* Observations Lithium burns slowly with a red flame.
Na with ‘hiss’ sound. Li A white solid is produced.
The colourless solution formed; turns red Lithium burns slowly with a red flame.
litmus paper to blue. Li A white / fume solid is produced. Sodium burns brightly with a yellow
Potassium moves vigorously on water flame.
Na
K surface with ‘hiss’ sound. Sodium burns brightly with a yellow A white solid is produced.
The colourless solution formed; turns red flame. Potassium burns very bright with a
Na
litmus paper to blue. A white solid is produced. purple flame.
K
Potassium burns very bright with a A white solid is produced.
Chemical equation: purple/lilac flame.
K
A white solid is produced.
Chemical Equation
2Li + 2H2O 2LiOH + H2
2Na + 2H2O 2NaOH + H2 Chemical equation
2K + 2H2O 2KOH + H2
2Li + O2 2Li2O 2Li + Cl2 2LiCl2
4Na + O2 2Na2O 2Na + Cl2 2NaCl2
PREPARED BY SULAIMAN SMKSM
4K + O2 2K2O 2K + Cl2 2KCl2](https://image.slidesharecdn.com/ptpublish-110512200312-phpapp01/85/Pt-publish-7-320.jpg)
