The document provides information about Nuclear Magnetic Resonance (NMR) Spectroscopy, including:
1. A brief history of NMR and important contributors such as Felix Bloch, Edward Purcell, Kurt Wuthrich, and Richard Ernst.
2. Applications of NMR including chemical structure analysis, material characterization, study of dynamic processes, and biomolecular structure determination.
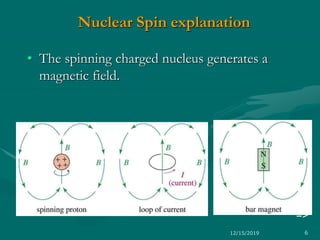
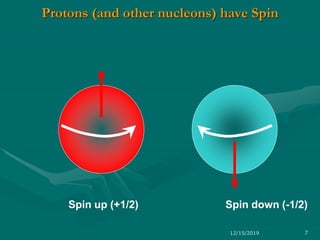
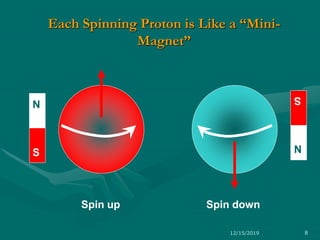
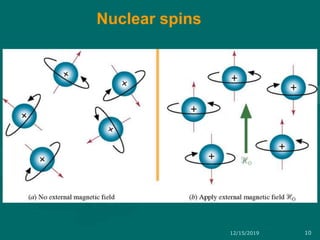
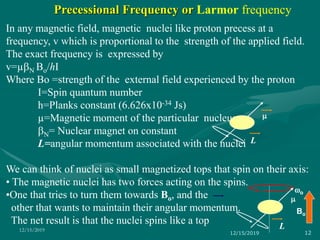
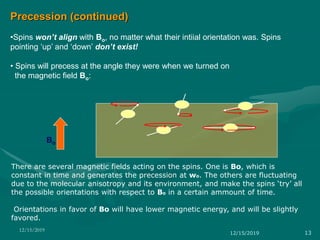
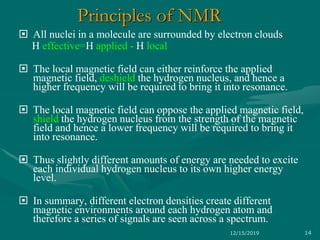
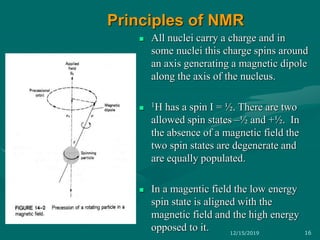
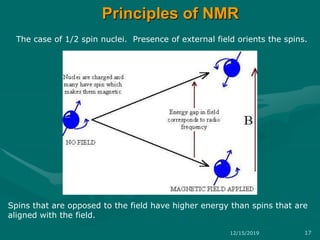
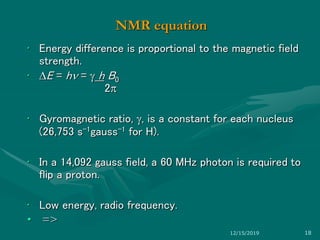
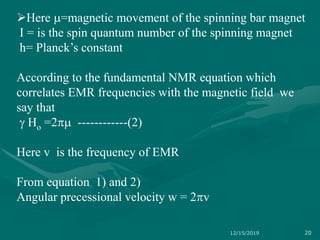
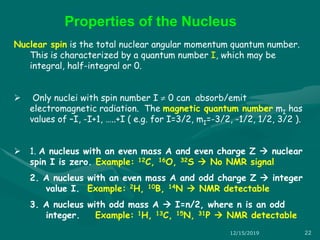
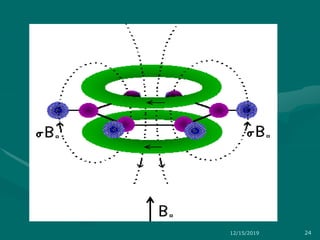
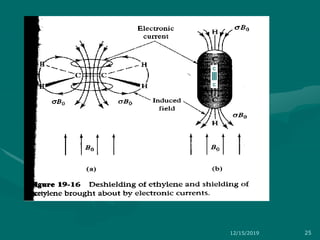
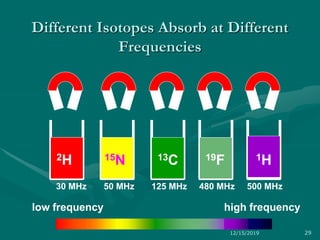
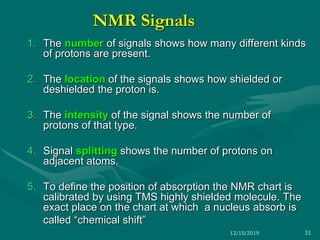
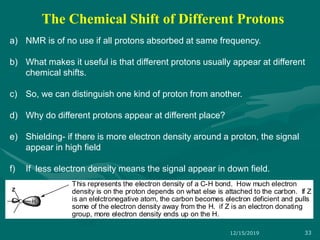
3. Explanations of key NMR concepts such as nuclear spin, precession, resonance frequency, and chemical shift.

















![12/15/2019
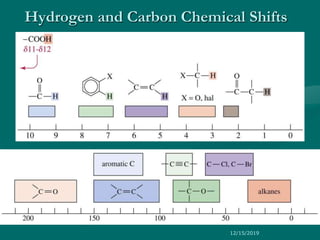
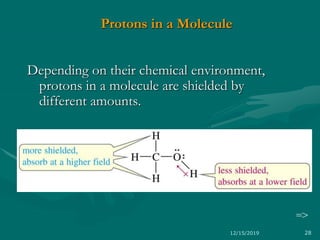
The chemical shift (δ) is defined as the difference between
the resonance position of a nucleus and that of a standard
reference compound.
Chemical shift (δ) =[Δν (Hz)/Applied resonance
frequency × 106 Hz)] × 106 ppm
where, Δν = Difference in frequency (Hz) between
the observed signal and that of the standard.
Convention for δ : TMS assigned (δ = 0), values
for other protons are measured positively downfield.
In other words, increasing δ corresponds to
increasing de-shielding of the nucleus.](https://image.slidesharecdn.com/1hnmr17-191215090007/85/NMR-SPECTROSCOPY-17-03-17-37-320.jpg)