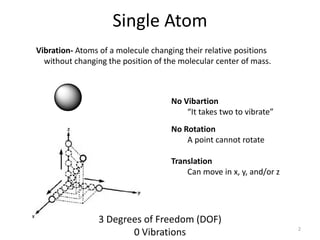
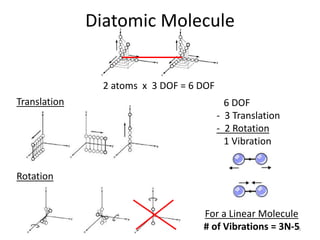
This document discusses vibrational spectroscopy and molecular vibrations. It begins by explaining how the degrees of freedom and vibrational modes are determined based on the number of atoms and molecular geometry. For a diatomic molecule there are 6 degrees of freedom - 3 translational and 2 rotational, with 1 vibrational mode. The number of vibrational modes is then calculated based on whether the molecule is linear or nonlinear. Several examples are then worked through, including calculating the vibrational modes of H2O using group theory. The key points covered are how vibrational spectroscopy can provide information about a molecule's internal motion and symmetry, and how group theory can be used to determine the number, symmetry and nature of vibrational modes.


















![Decomposition/Reduction Formula
h = 1 + 1 + 1 + 1 = 4
order (h)
aA1 = + (1)(-1)(1) + (1)(1)(1) + (1)(3)(1)
1
4
[ ]
(1)(9)(1) = 3
=
12
4
Example: H2O
G 9 3
-1 1
G = 3A1 + A2 + 2B1 + 3B2
21](https://image.slidesharecdn.com/vibrationalspectroscopy-221103140722-db7bcaaa/85/Vibrational-spectroscopy-pptx-21-320.jpg)










![Decomposition/Reduction Formula
h = 1 + 1 + 1 + 1 = 4
order (h)
aA1 = + (1)(0)(1) + (1)(0)(1) + (1)(2)(1)
1
4
[ ]
(1)(2)(1) = 1
=
4
4
Example: H2O
G 2 2
0 0
G = A1 + B2
aB2 = + (1)(0)(-1) + (1)(0)(-1) + (1)(2)(1)
4
(1)(2)(1) = 1
=
4
1
[ ] 4
32](https://image.slidesharecdn.com/vibrationalspectroscopy-221103140722-db7bcaaa/85/Vibrational-spectroscopy-pptx-32-320.jpg)














