Embed presentation
Downloaded 840 times




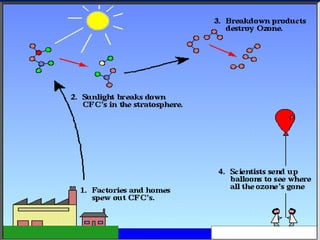
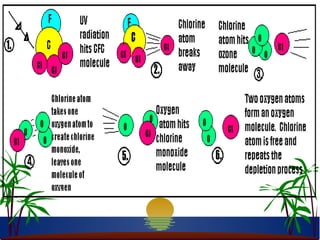


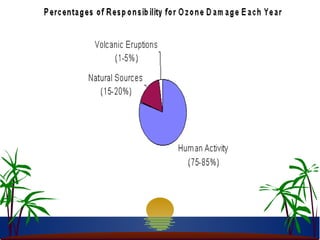

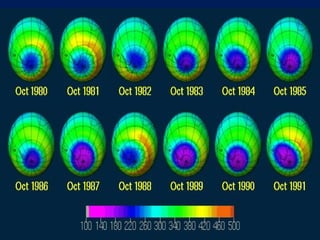
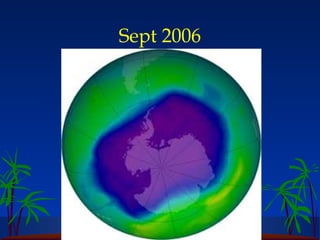
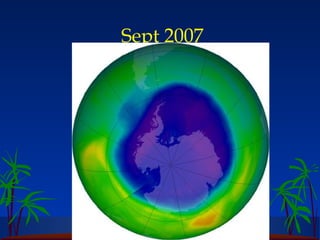



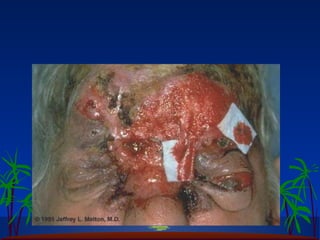



The ozone layer in the stratosphere absorbs most UV light from the sun. Ozone is formed when oxygen molecules are split by UV light or lightning, then join with other oxygen molecules to form ozone (O3). Chlorofluorocarbons (CFCs) used in refrigerants and air conditioners were depleting the ozone layer as they take 10-20 years to break down in the stratosphere, and each chlorine atom can destroy 100,000 ozone molecules. This was causing seasonal thinning of the ozone layer over Antarctica, known as the ozone hole. Thinning of the ozone layer allows more harmful UV-B radiation to pass through, which can damage DNA and



















