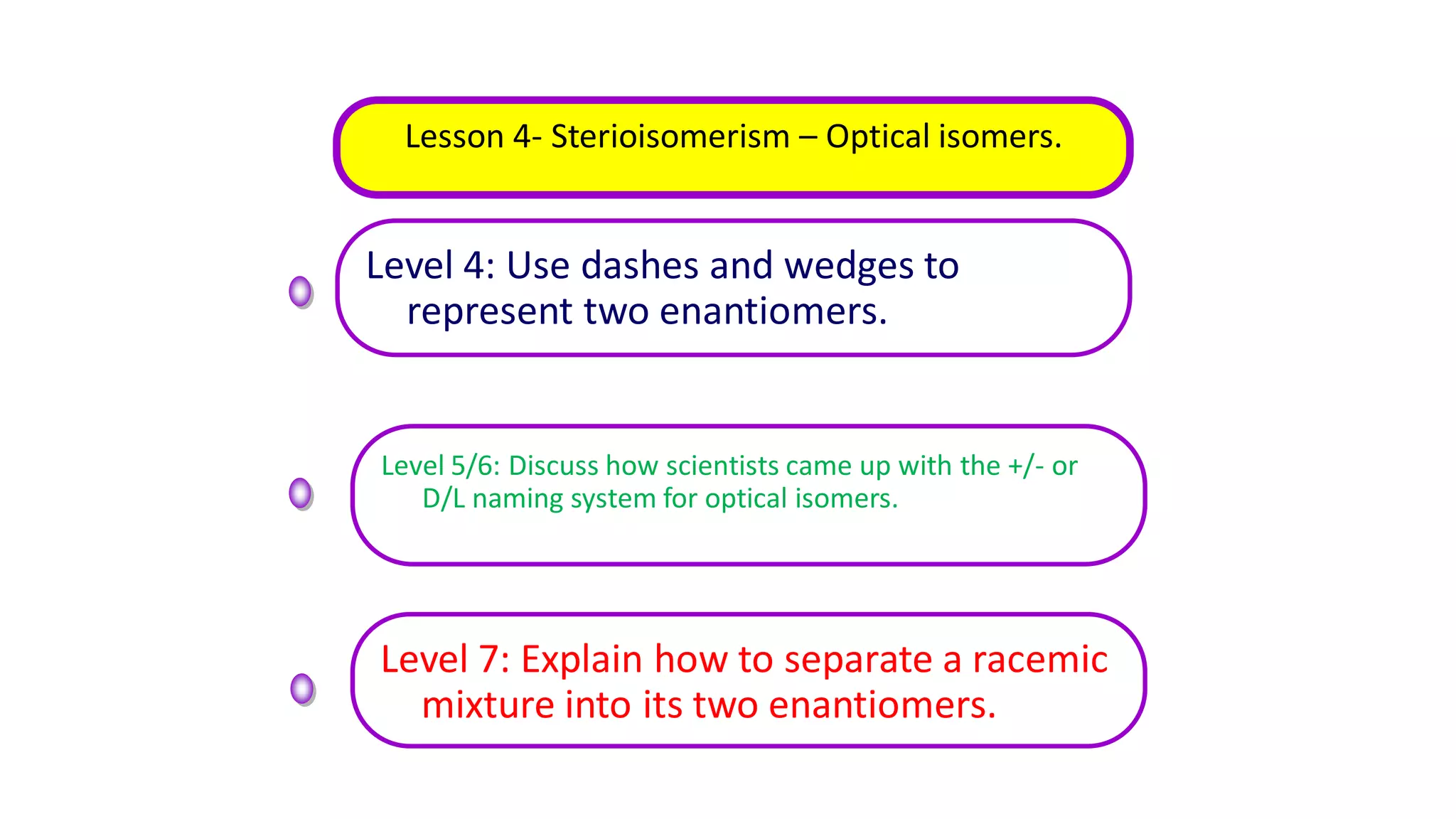
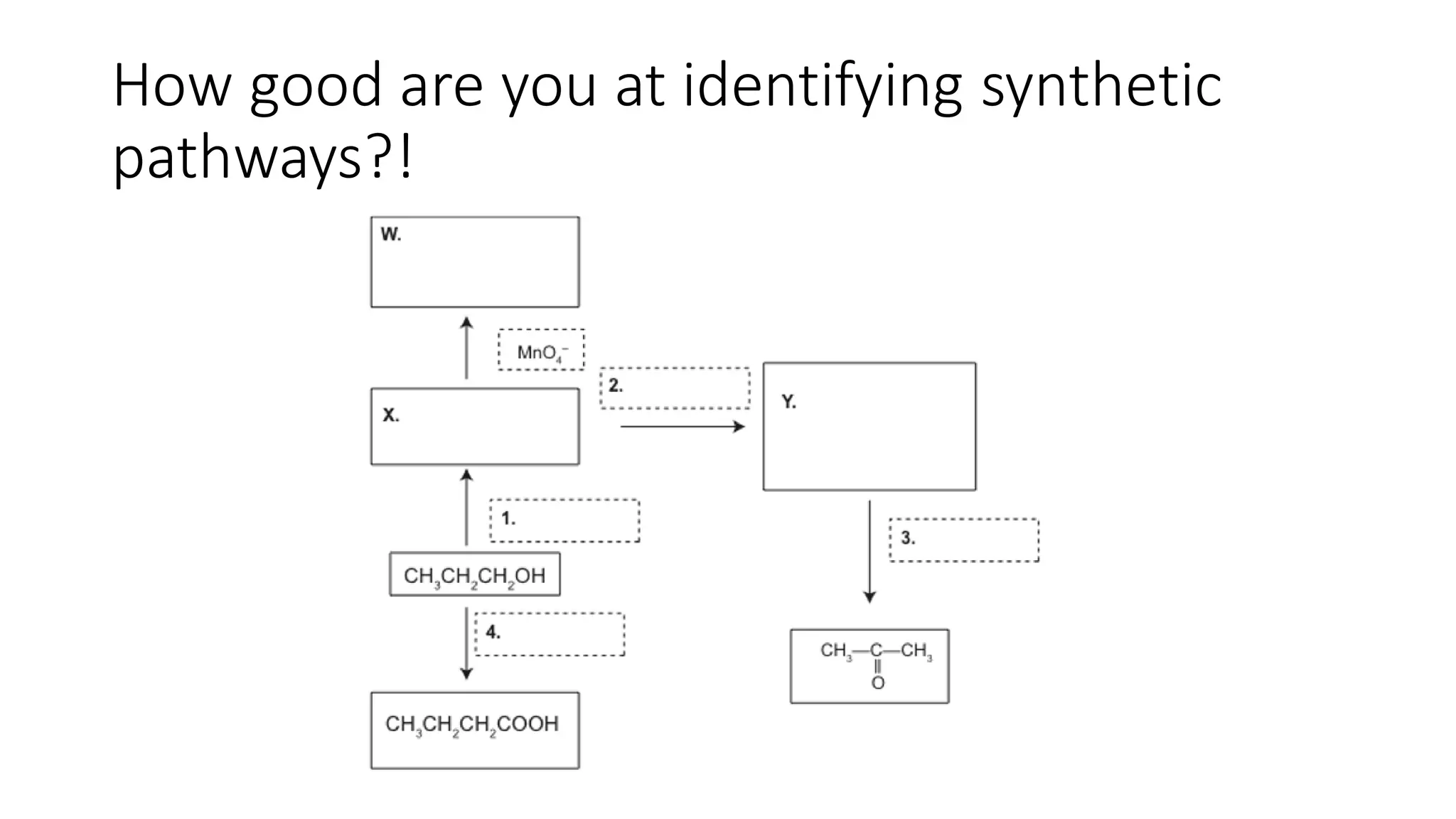
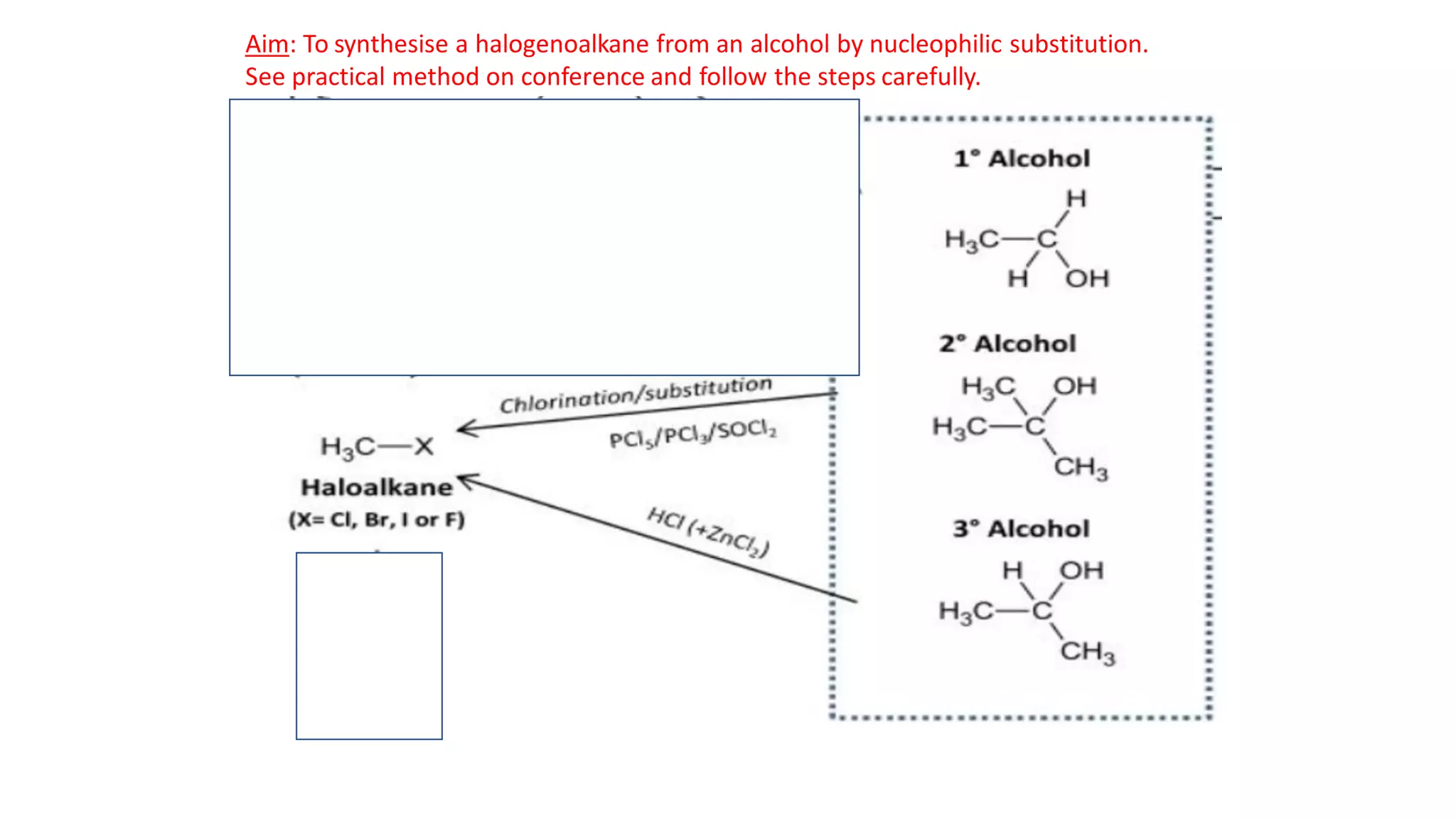
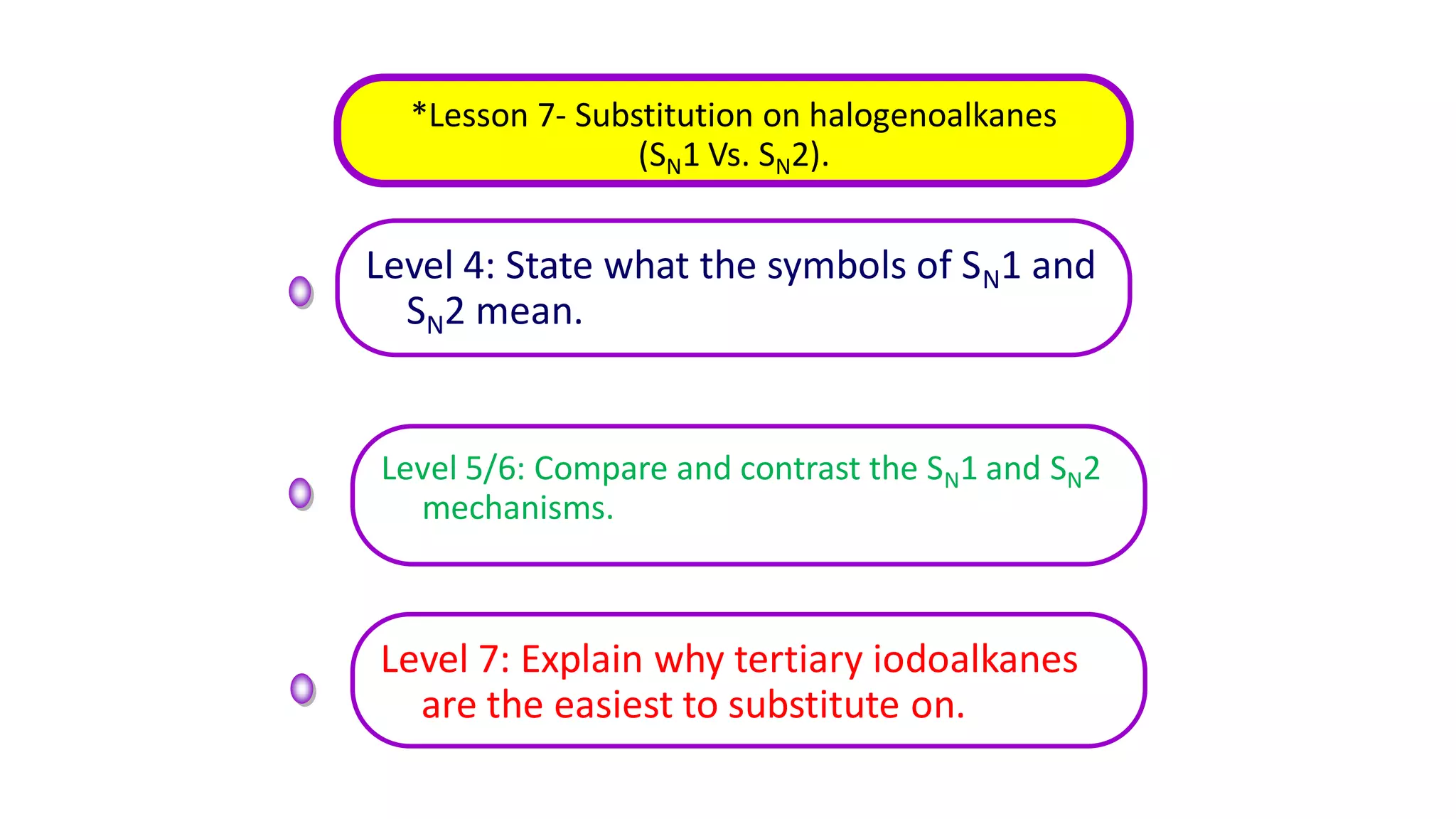
This document outlines lessons for a 3 week organic chemistry topic. It covers fundamentals of organic chemistry, stereoisomerism including cis/trans, E/Z and optical isomers. It also covers organic reaction types like substitution, addition and oxidation/reduction. Specific lessons cover nucleophilic substitution mechanisms SN1 and SN2, addition reactions to alkenes and benzene, and separating optical isomers. Activities include naming organic compounds, drawing 3D isomer structures, and working through reaction mechanisms and practice questions.

















































![2 things making alkenes so reactive…..
1. Sp2 hybridised carbons (e.g. in ethene) giving it a trigonal planar
shape which is more open to attack than if it was tetrahedral (sp3)
[Note: ethyne is sp hybridised, has a triple bond, linear shape which
is even more attractive to electrophiles!]
2. The π bond between the unhybridized pz orbitals of the two
carbons is above and below the plane of the bond axis. This makes
the two electrons in it less associated/attracted to the carbon nuclei
and so easier to break during addition reactions than the σ bond.](https://image.slidesharecdn.com/t20-rhu2017-180427221929/75/T20-IB-Chemistry-Organic-59-2048.jpg)