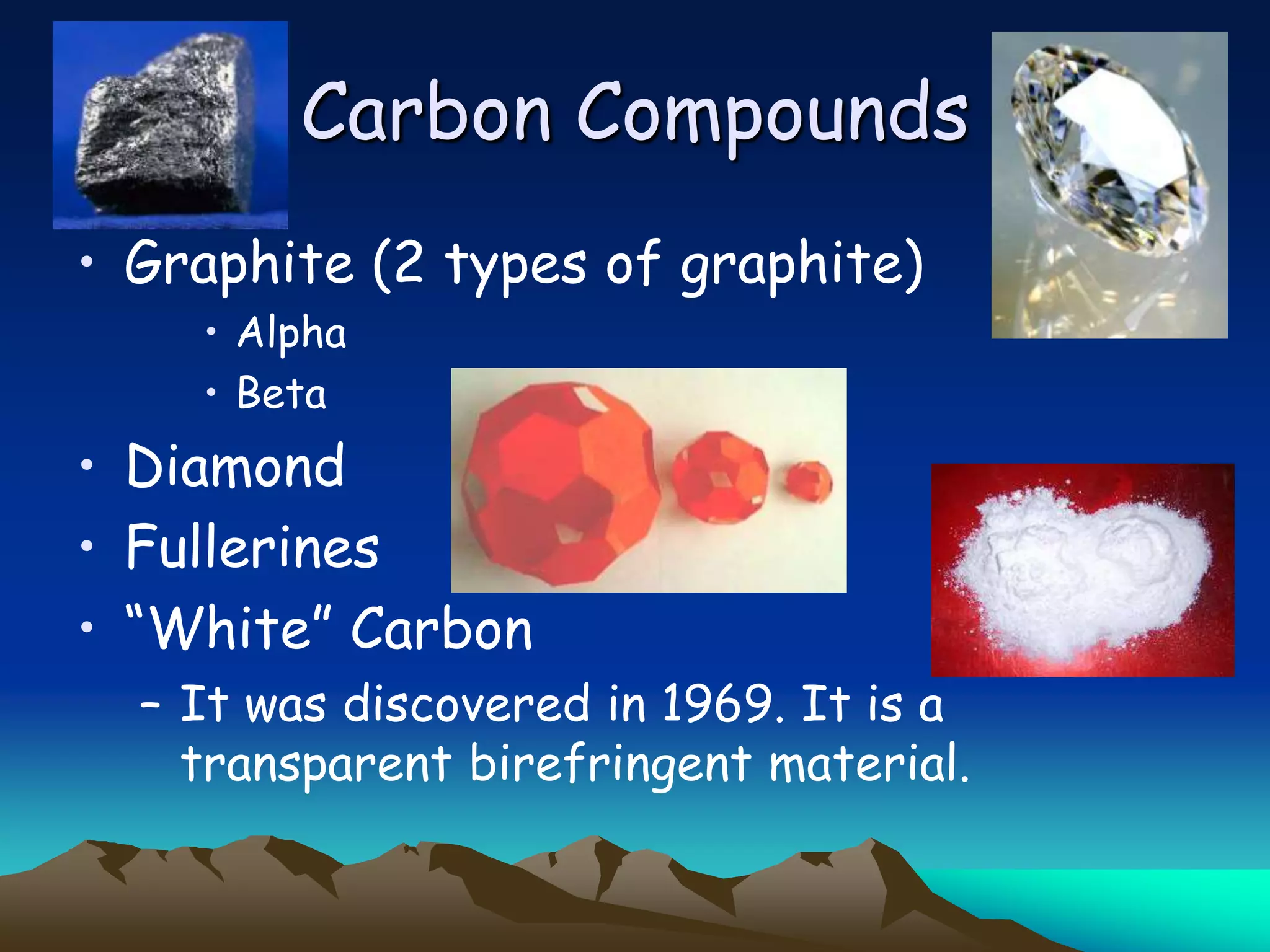
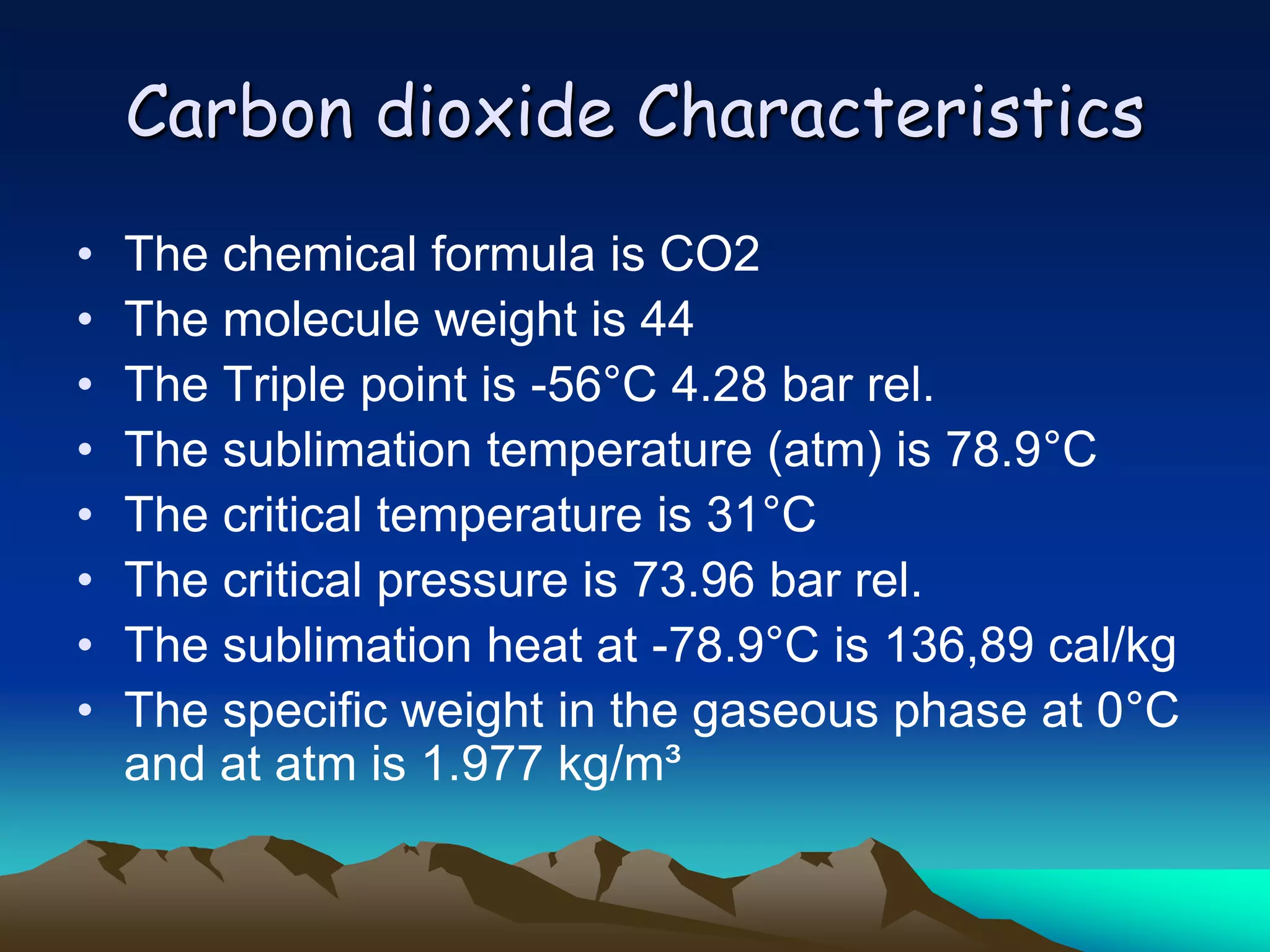
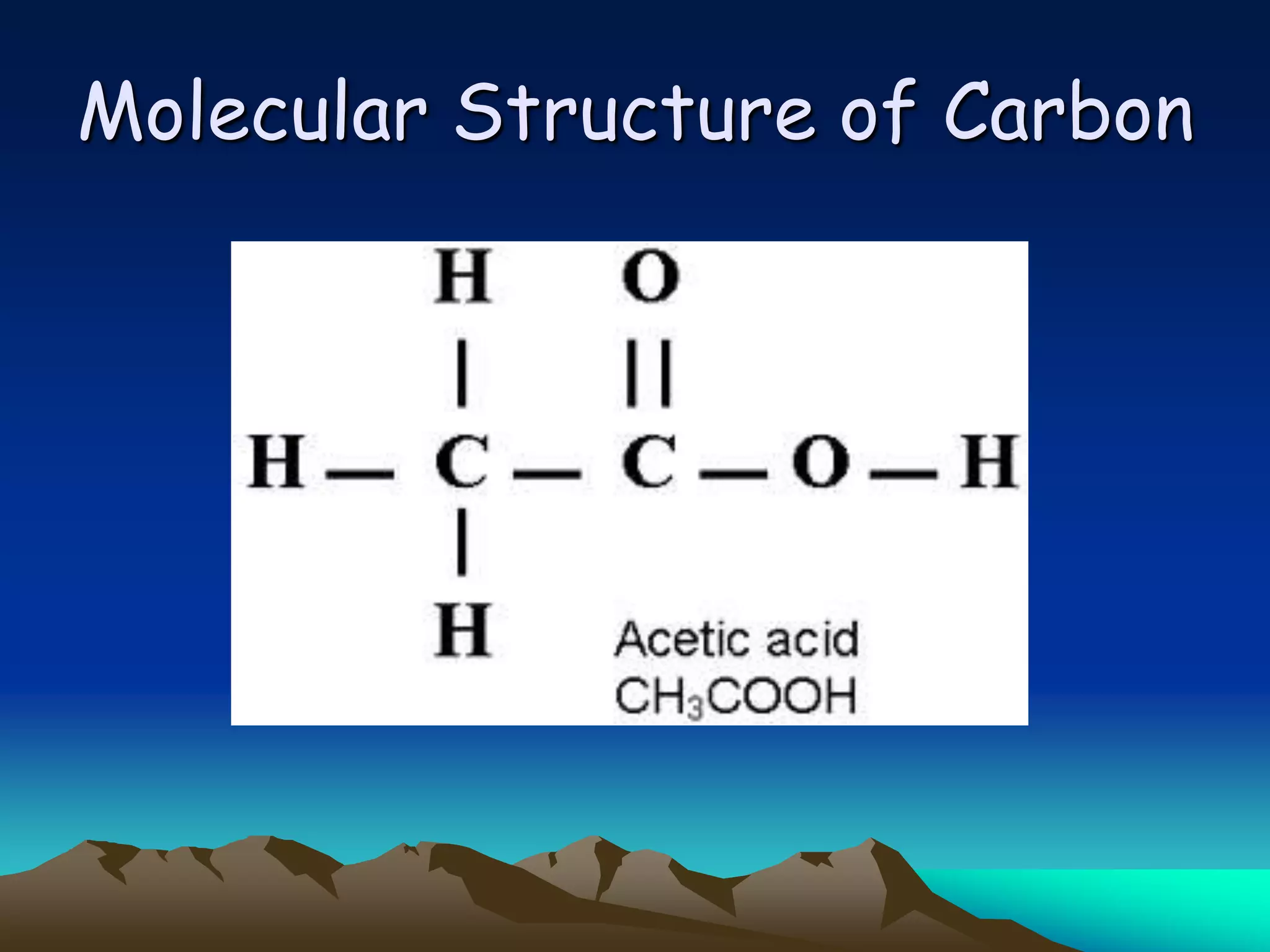
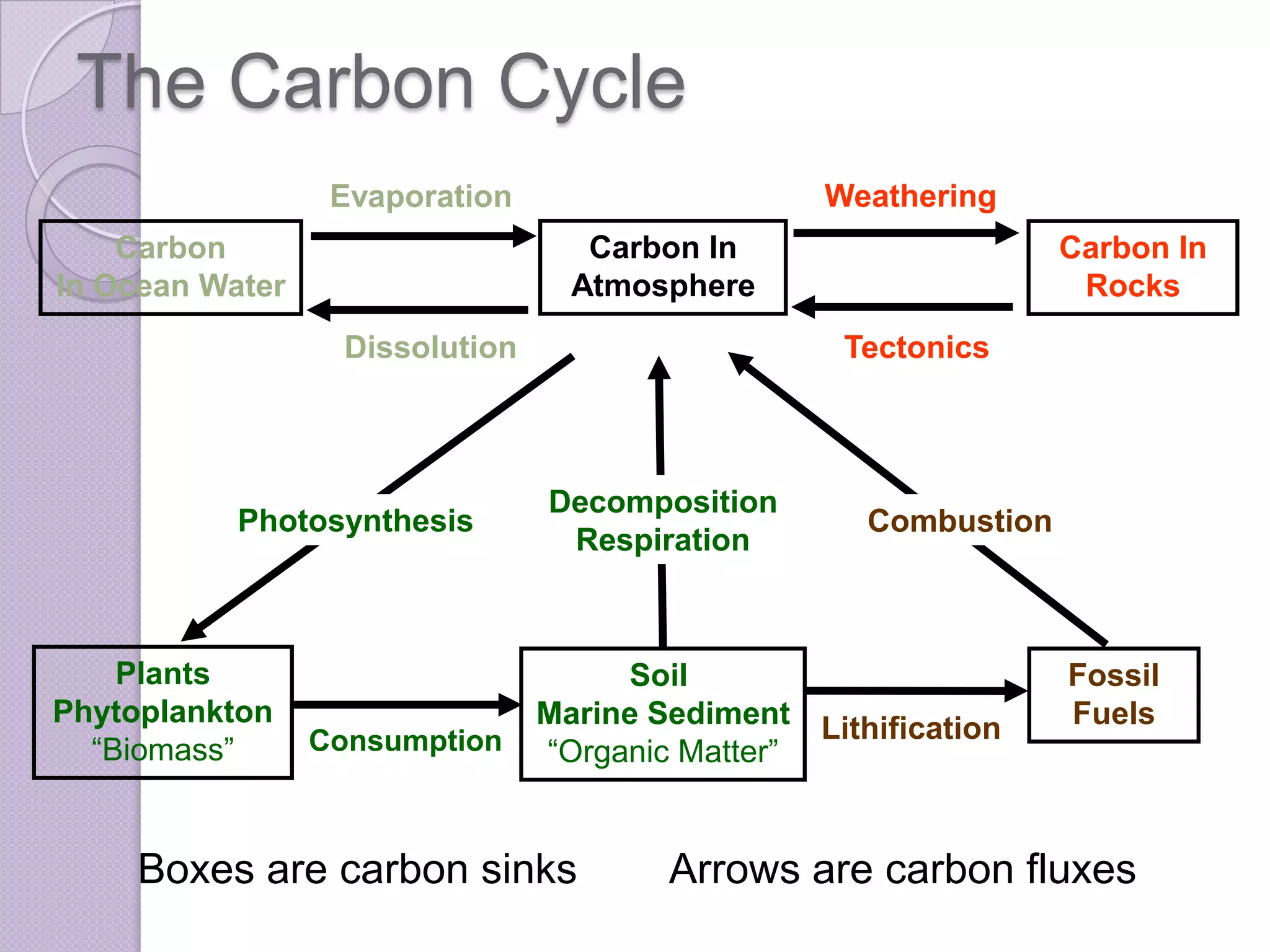
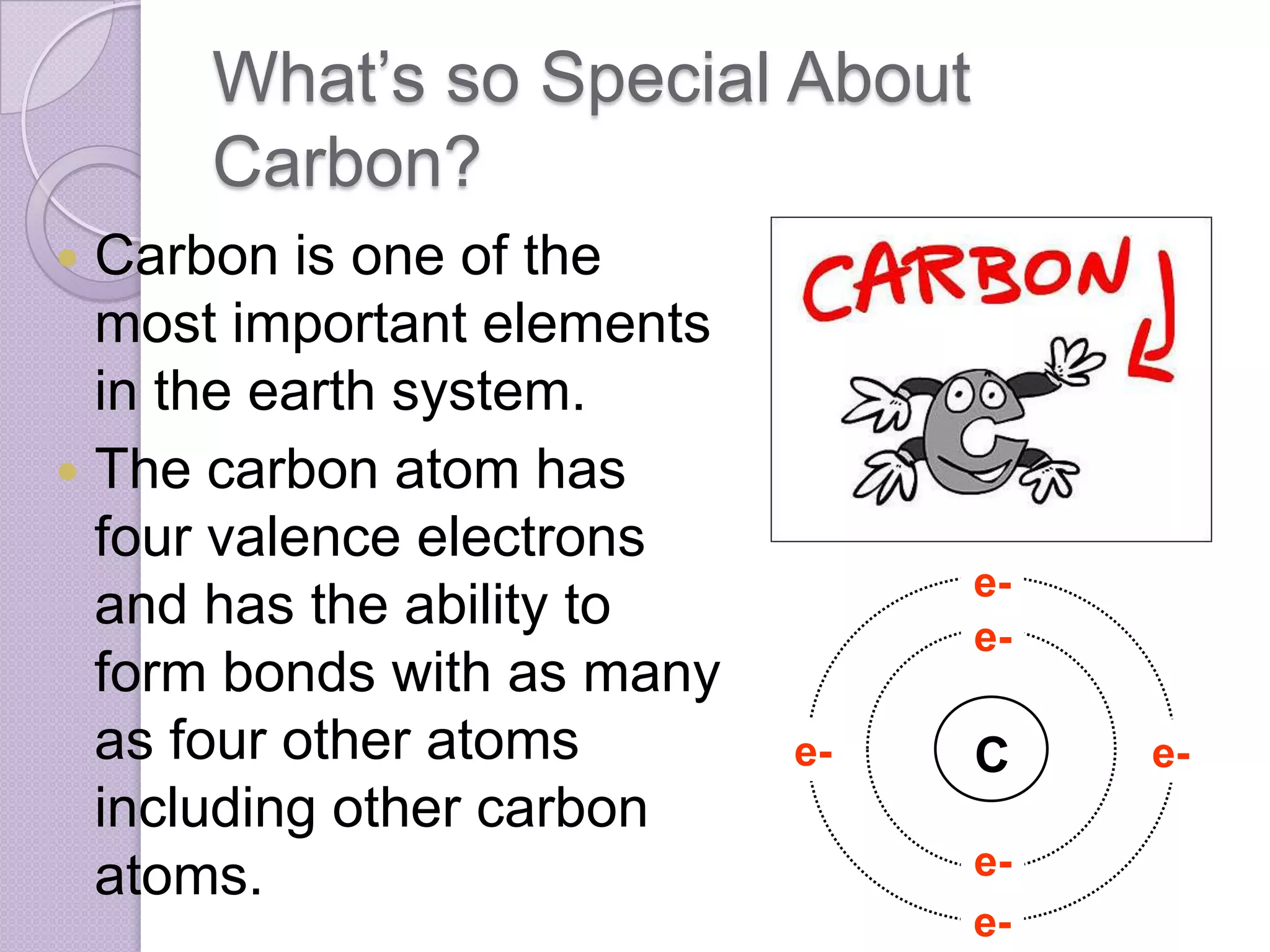
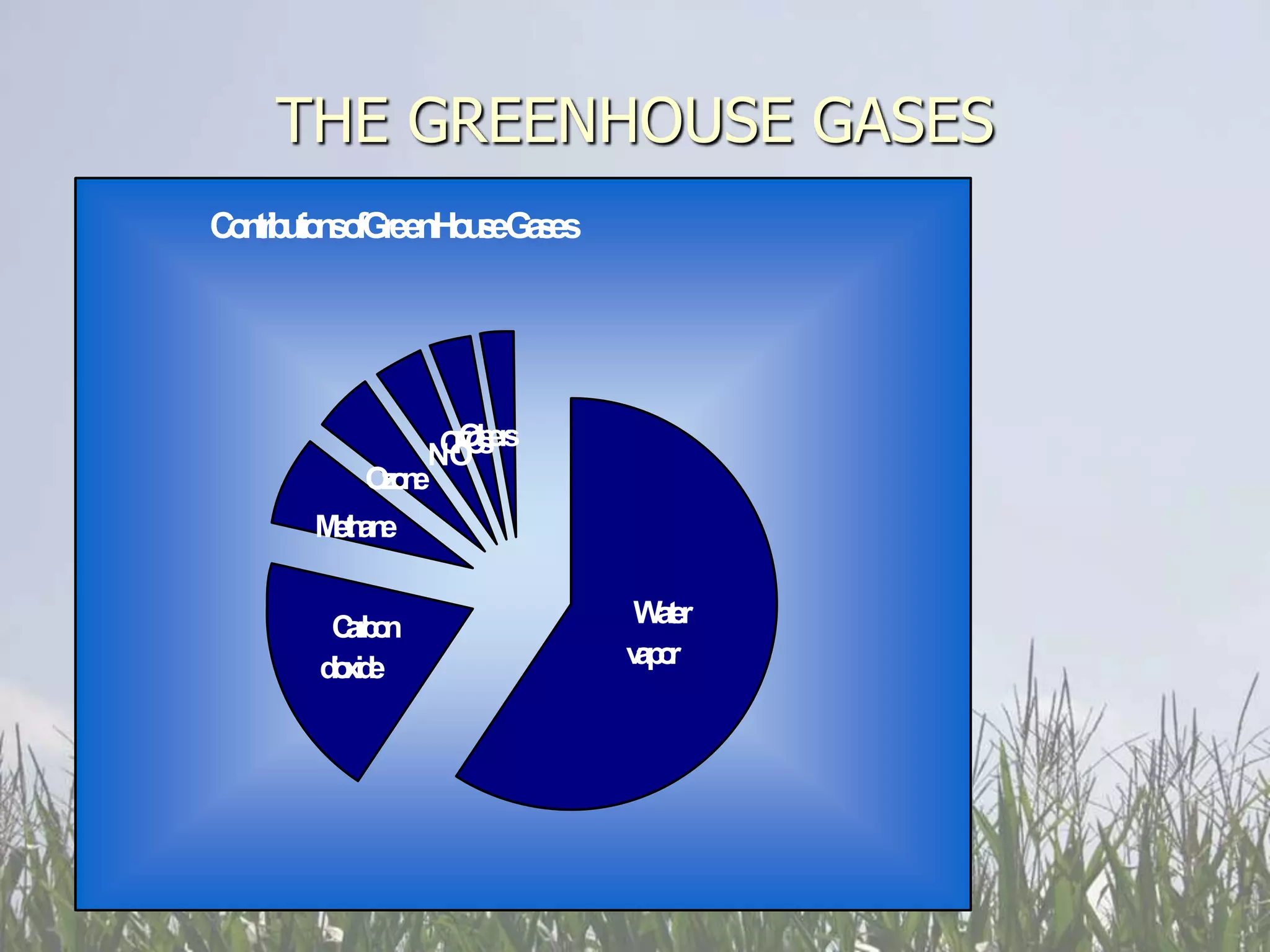
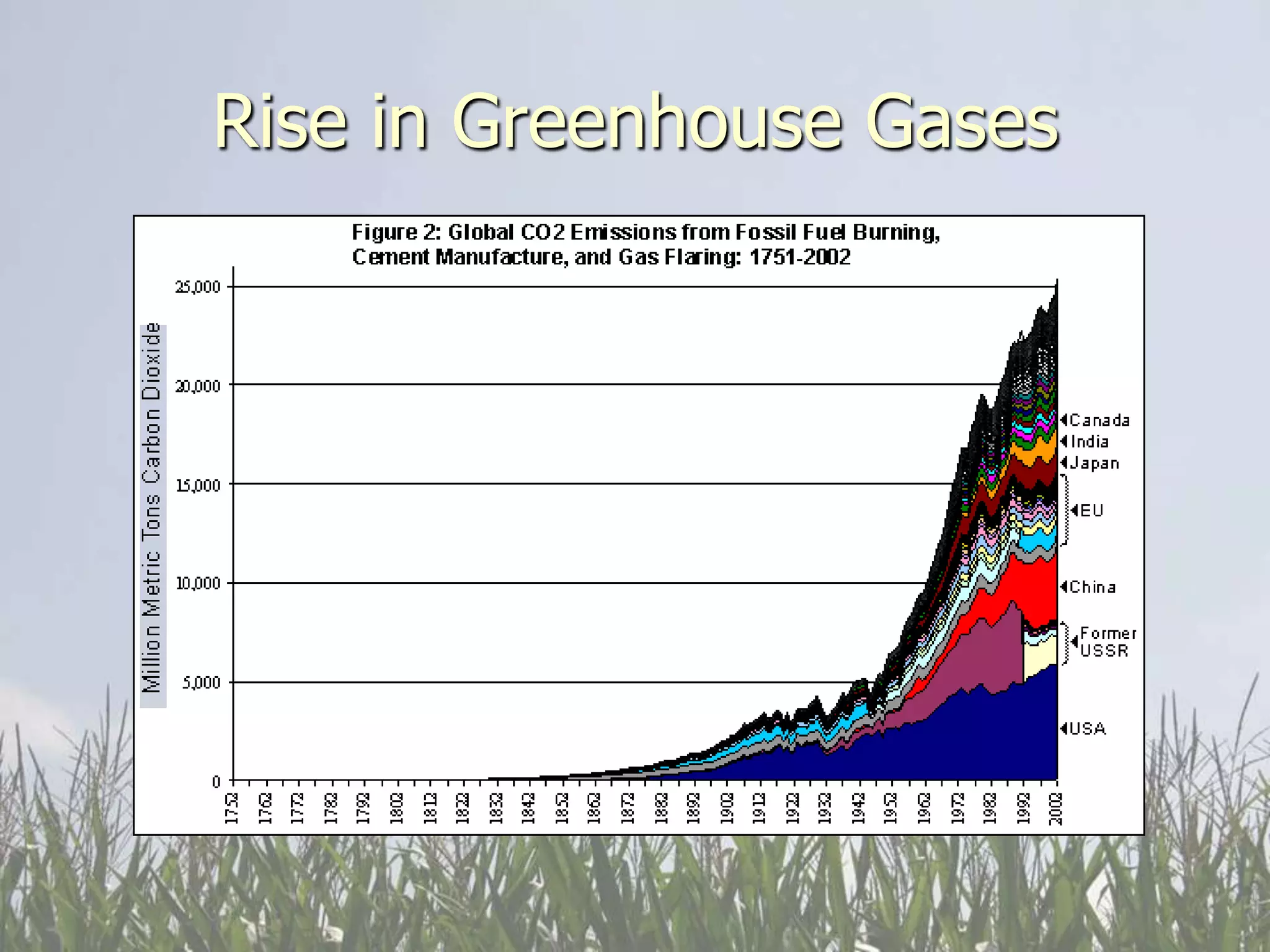
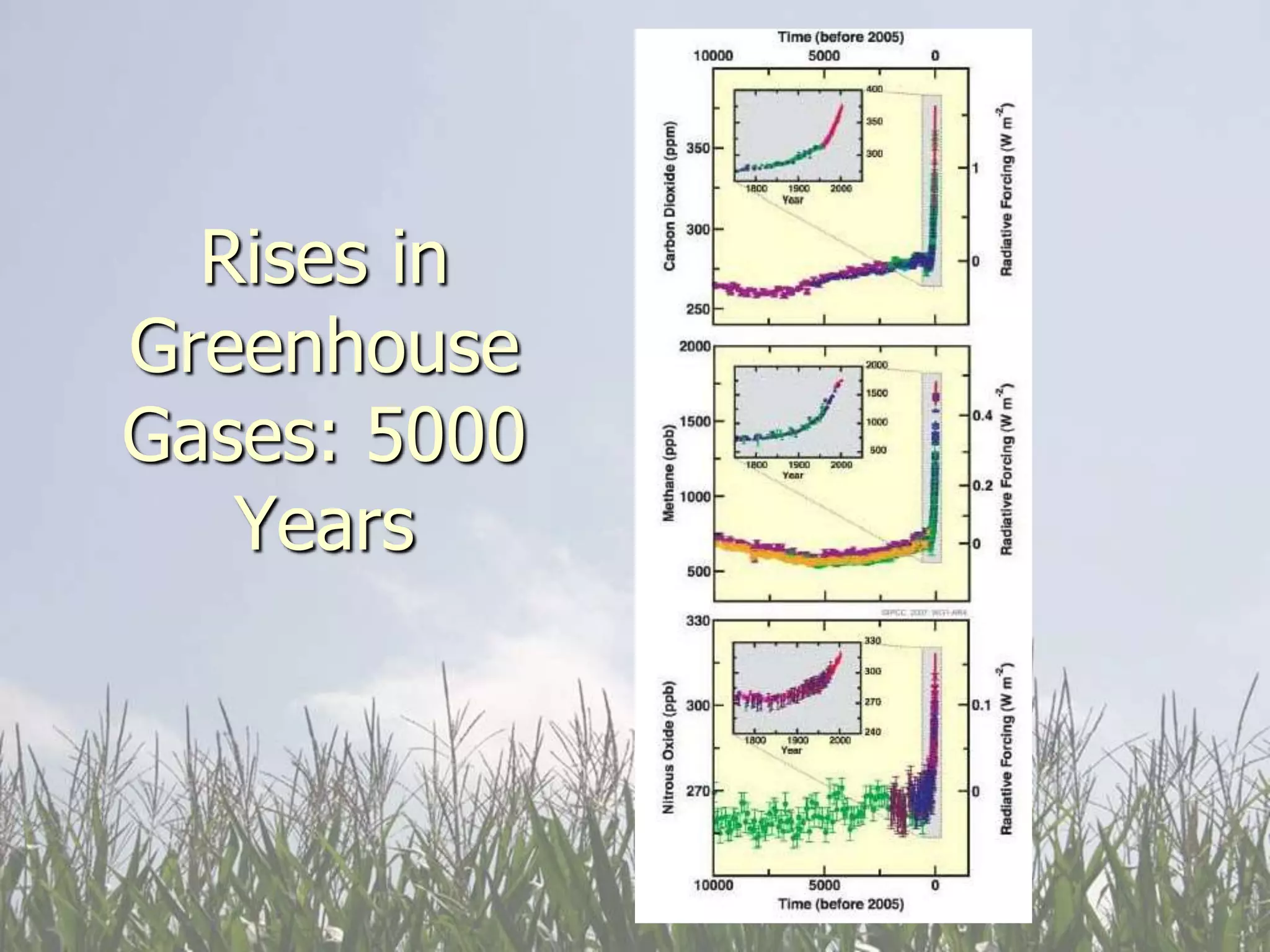
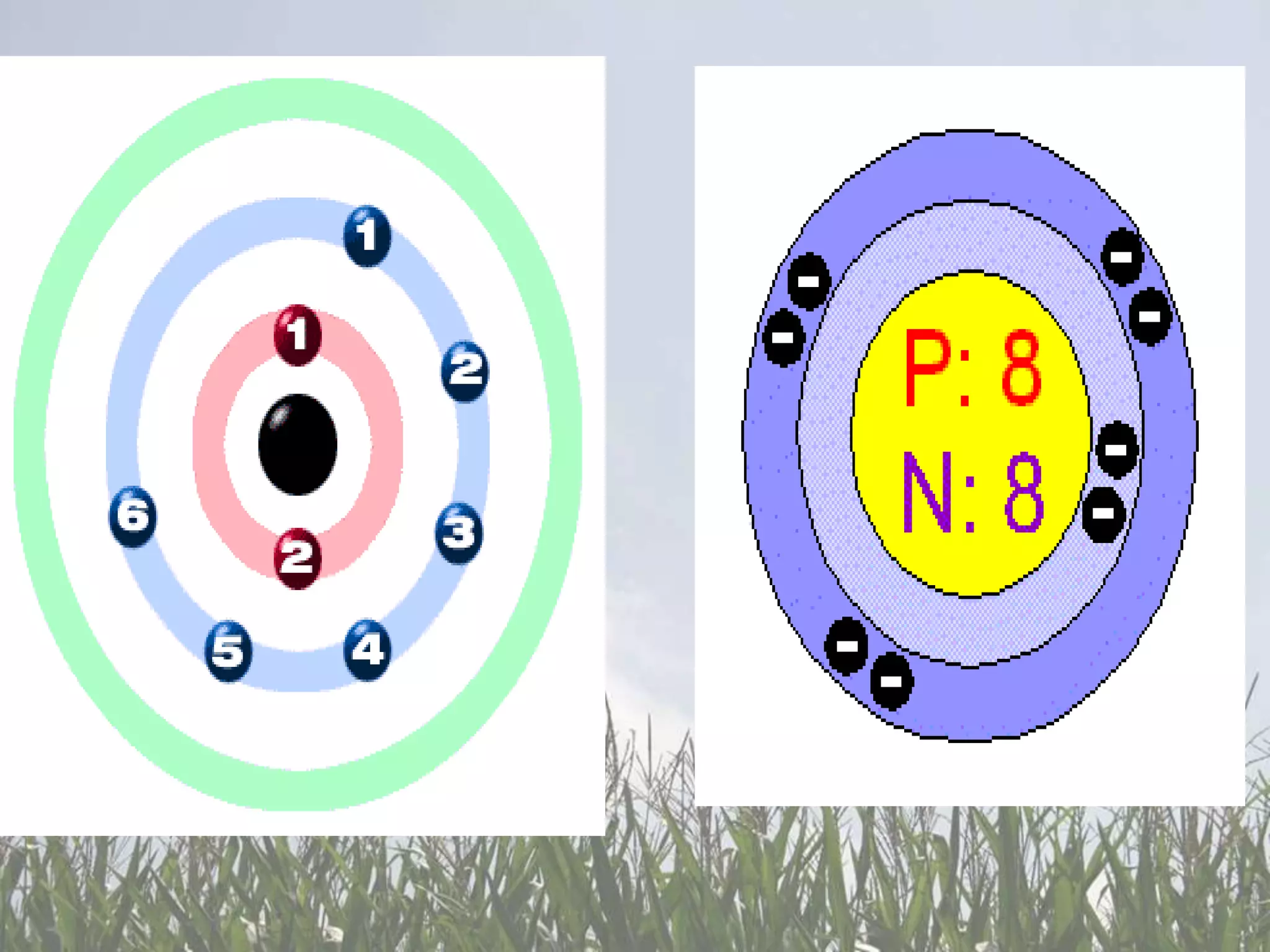
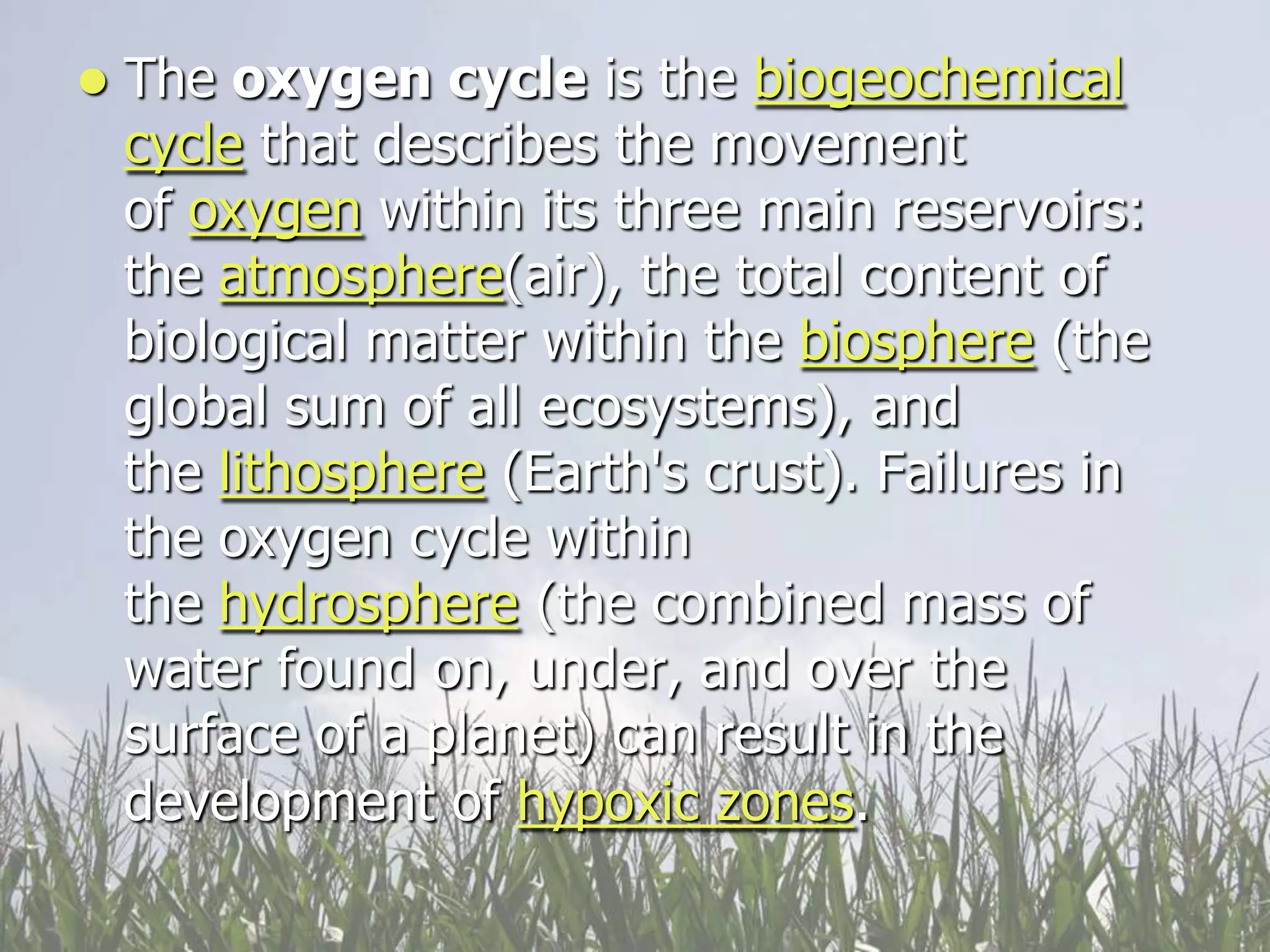
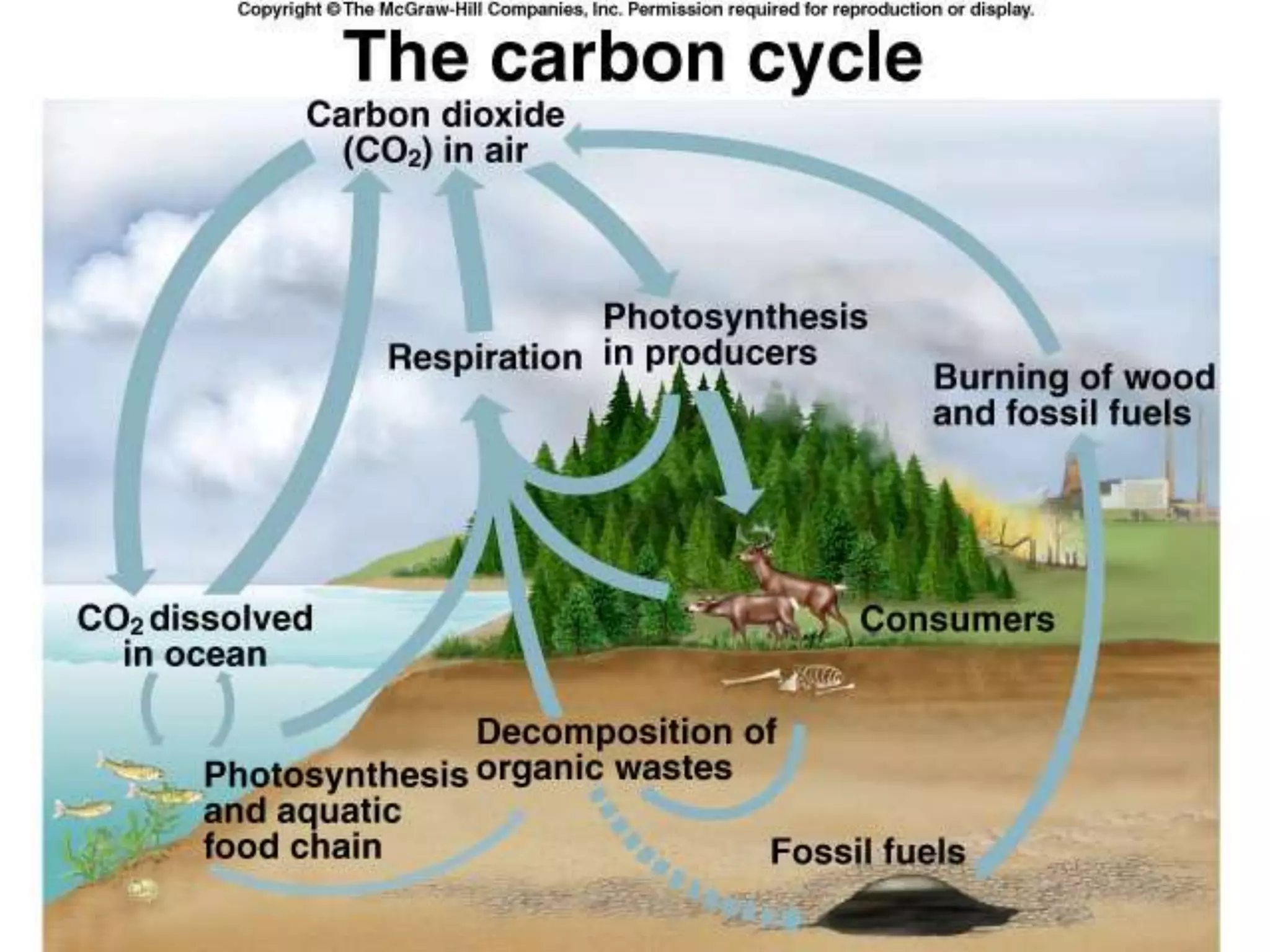
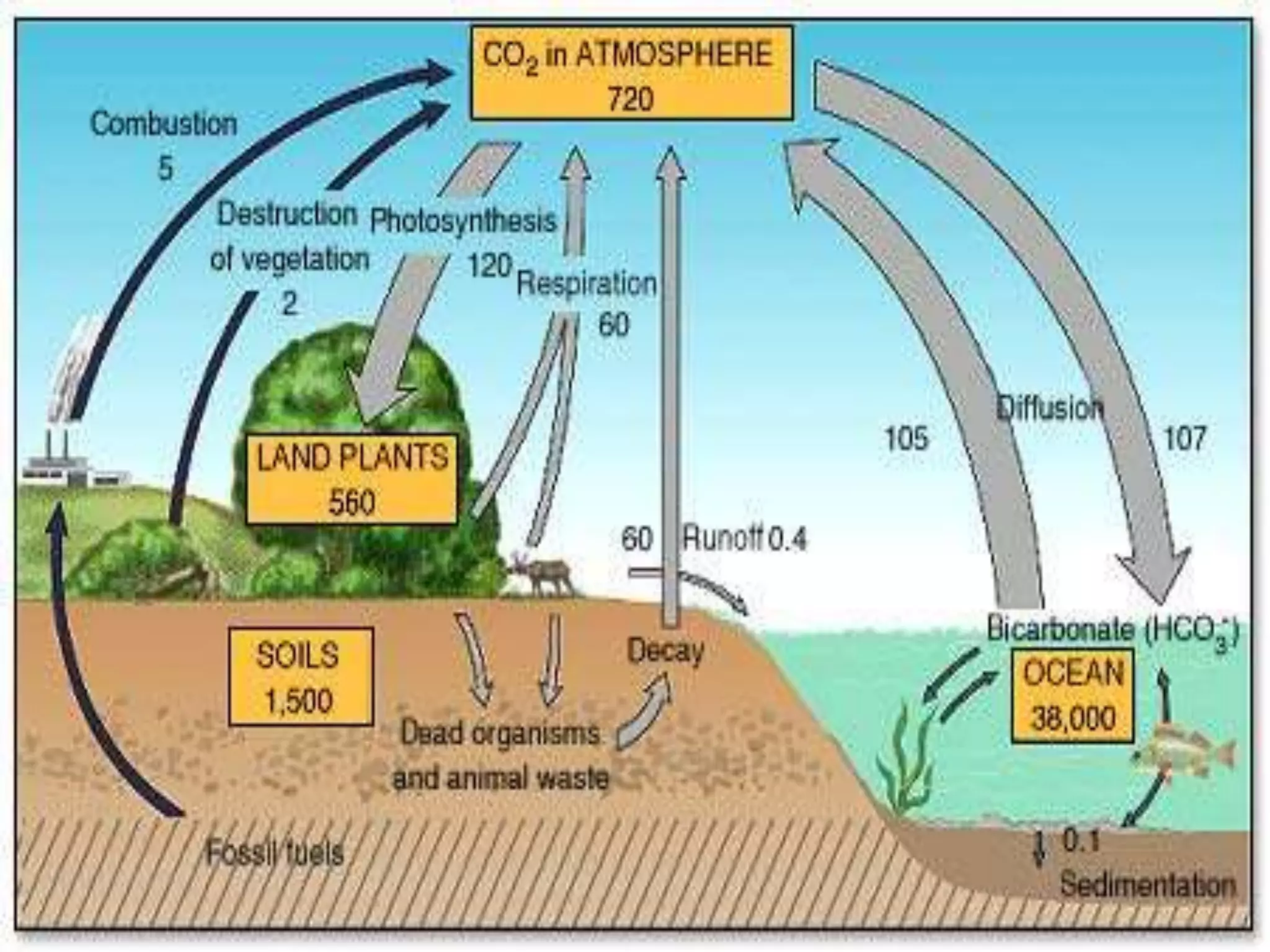
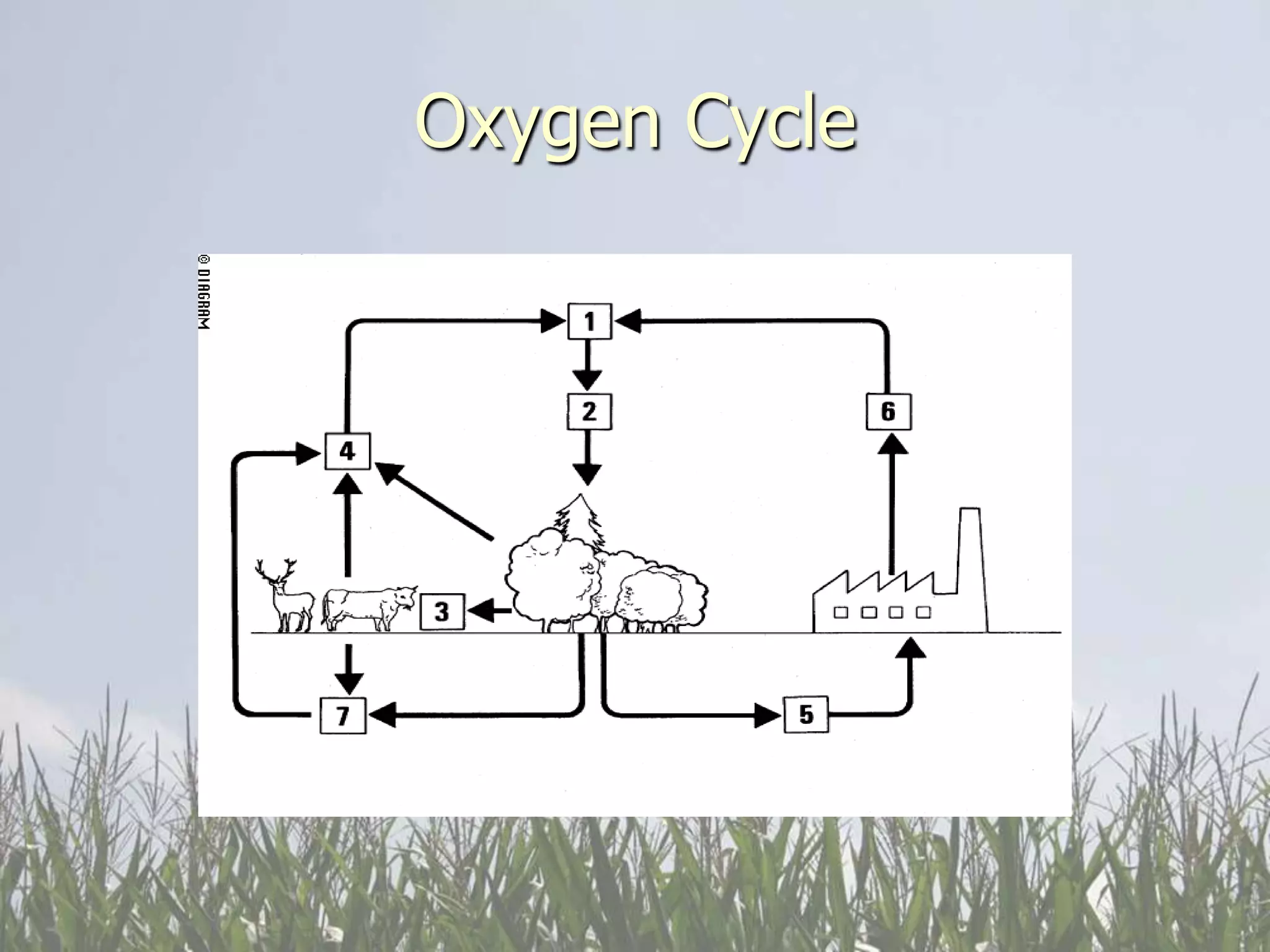
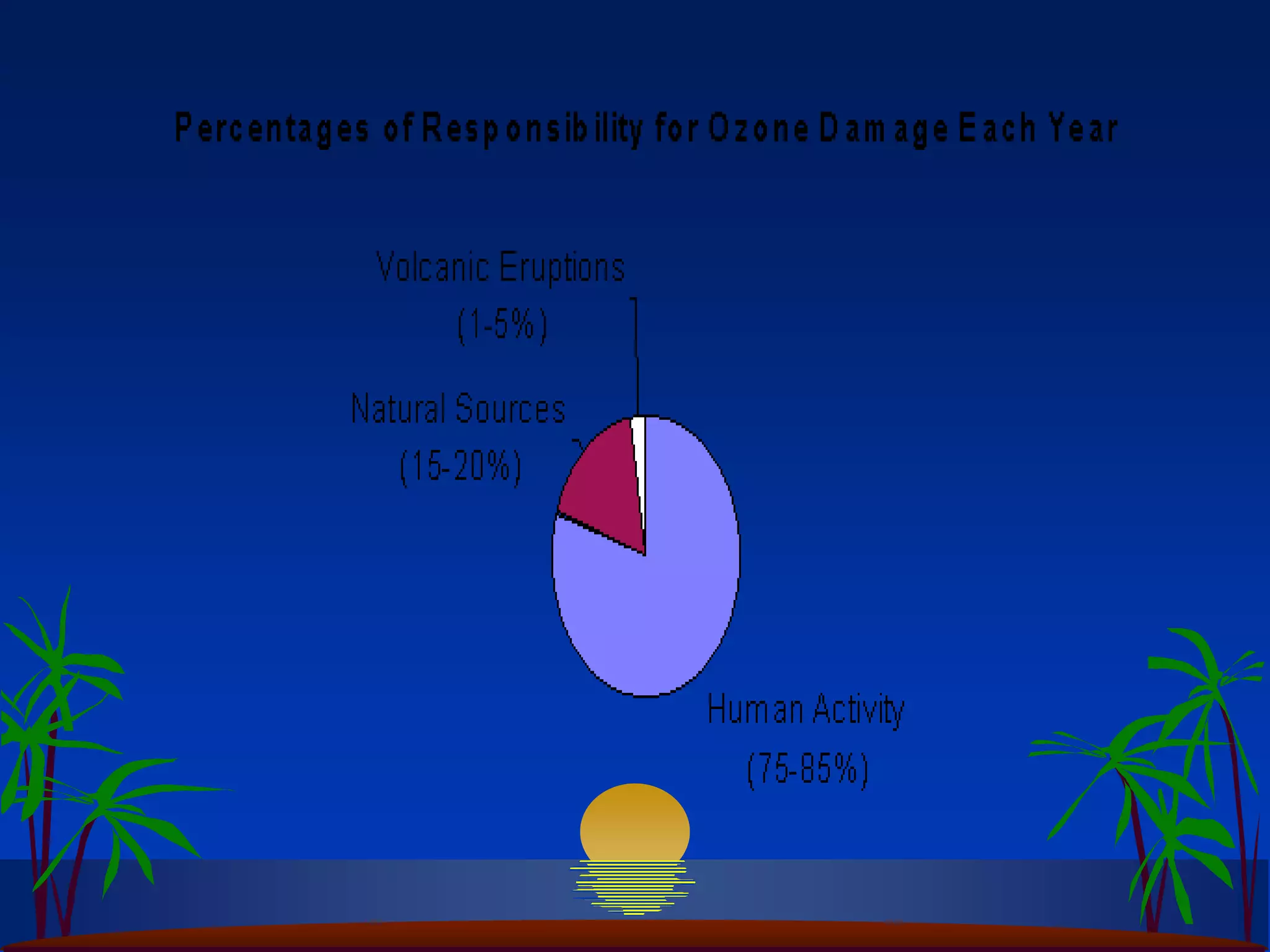
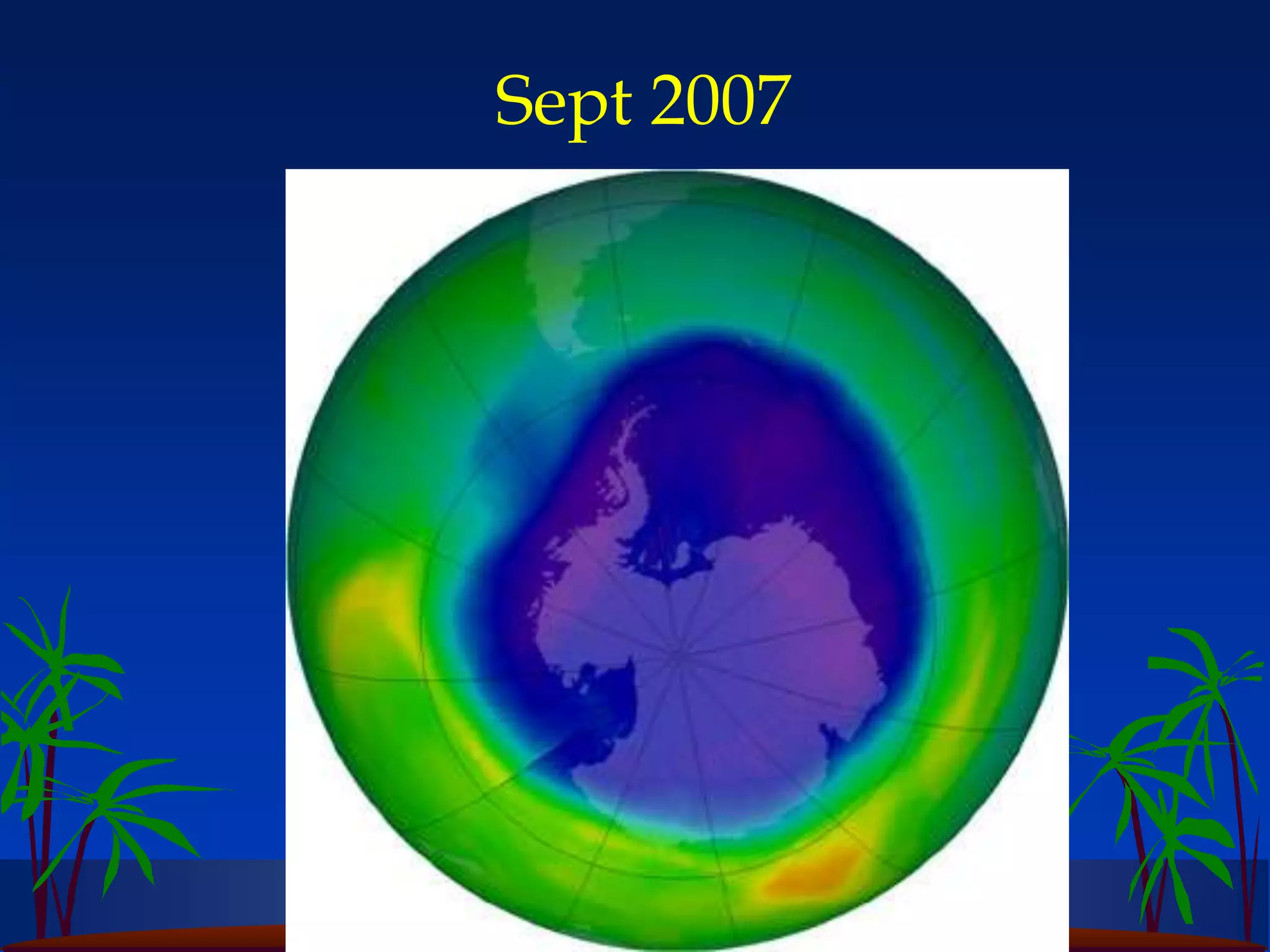
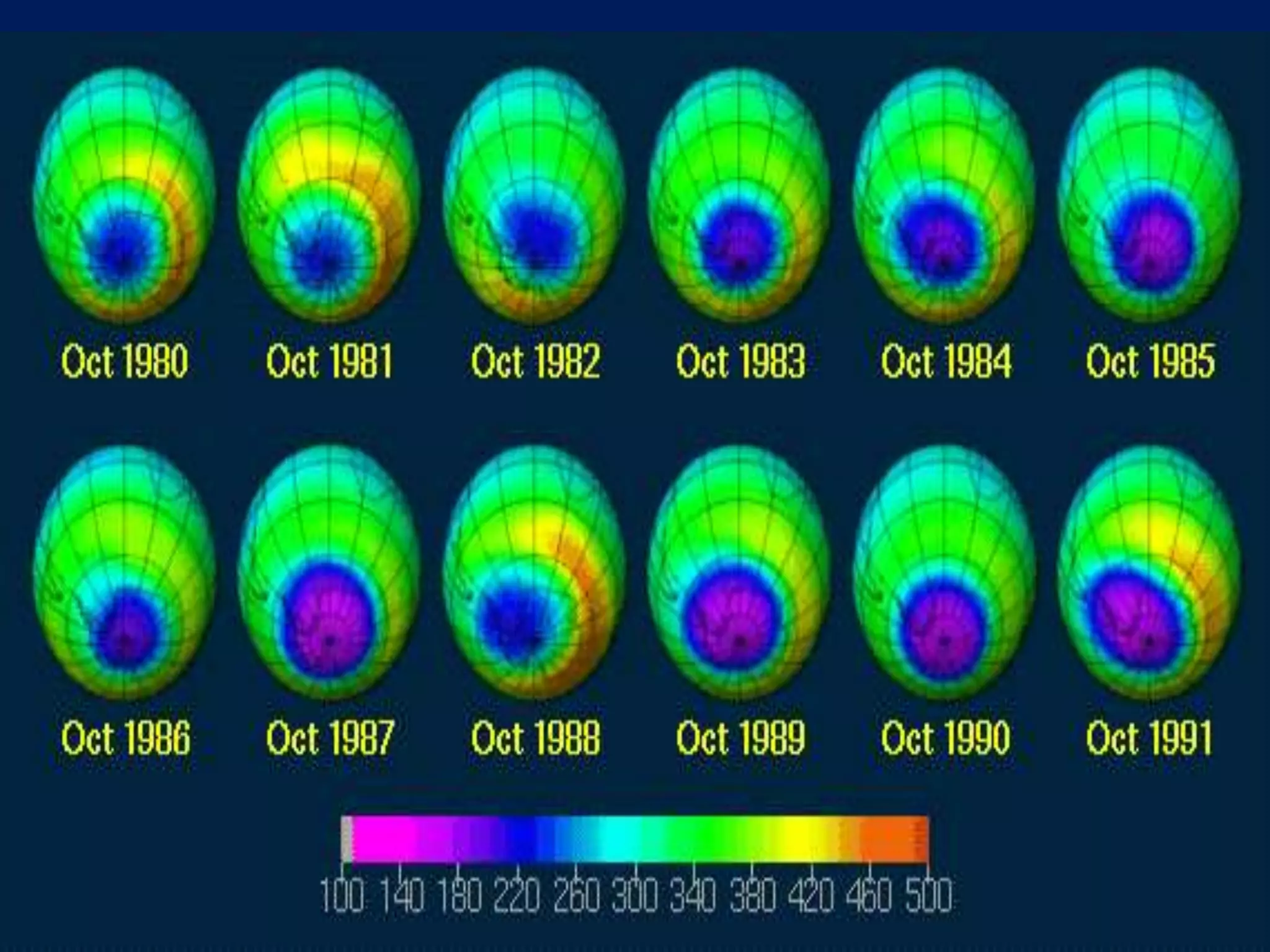
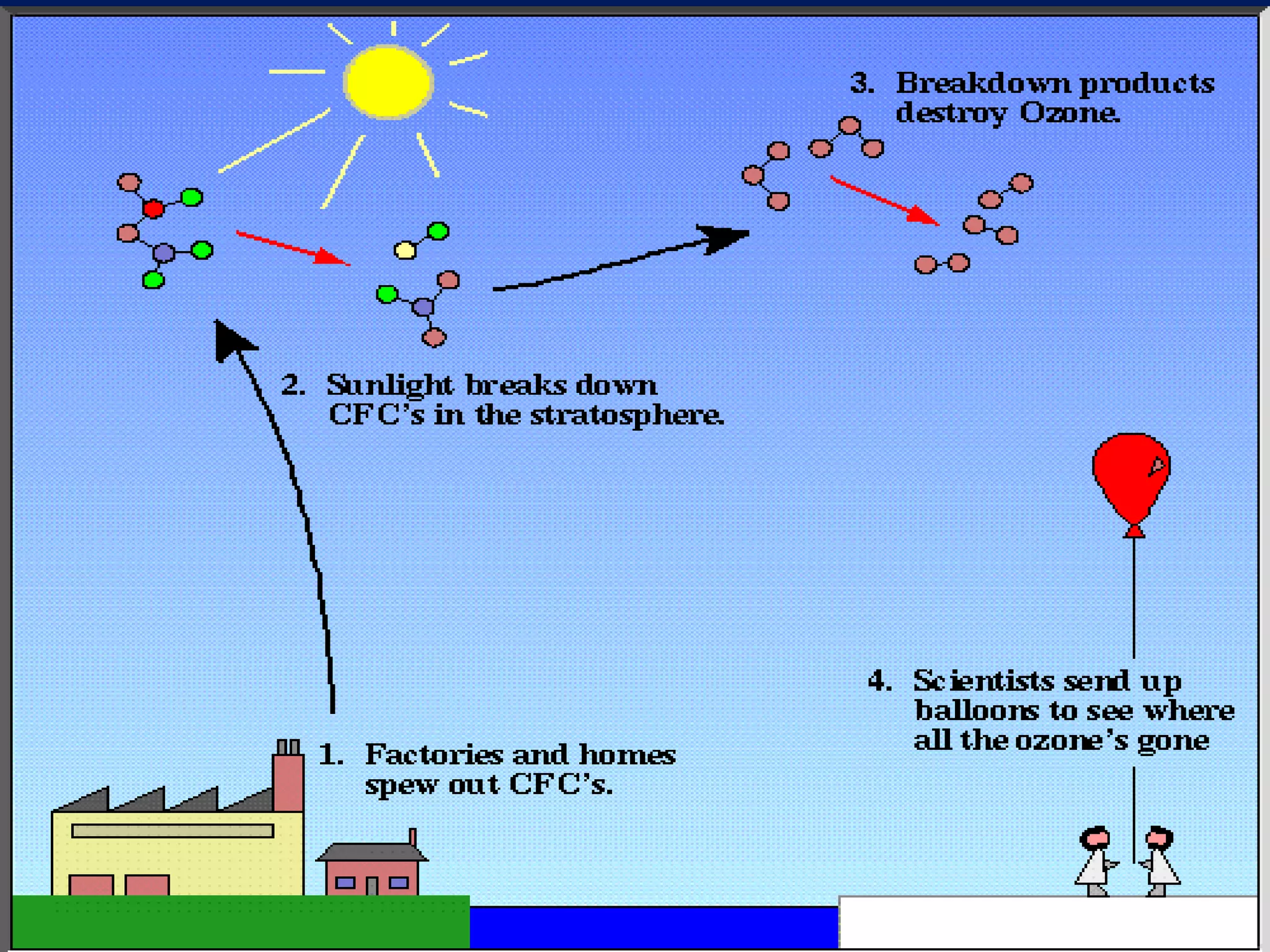
Carbon and oxygen are two of the most abundant elements on Earth. Carbon is found in all living things and is the basis of organic molecules. It cycles continuously between the atmosphere, living things, oceans, and geologic reservoirs. Oxygen is produced through photosynthesis and is essential for cellular respiration. It cycles between the atmosphere, biosphere, and lithosphere through natural processes. Disruptions to these elemental cycles can impact climate and ecosystems.



![Electron Configuration Diagrams
• Carbon has 6 electrons, 6
neutrons, and 6 protons.
• The electron
configuration of carbon is
[He] 2s2 2p2
• The atomic radius is 77
pm or 0.91Å
• Its oxidation states are 4
and 2](https://image.slidesharecdn.com/pptpresentation-130107101643-phpapp02/75/Ppt-presentation-4-2048.jpg)