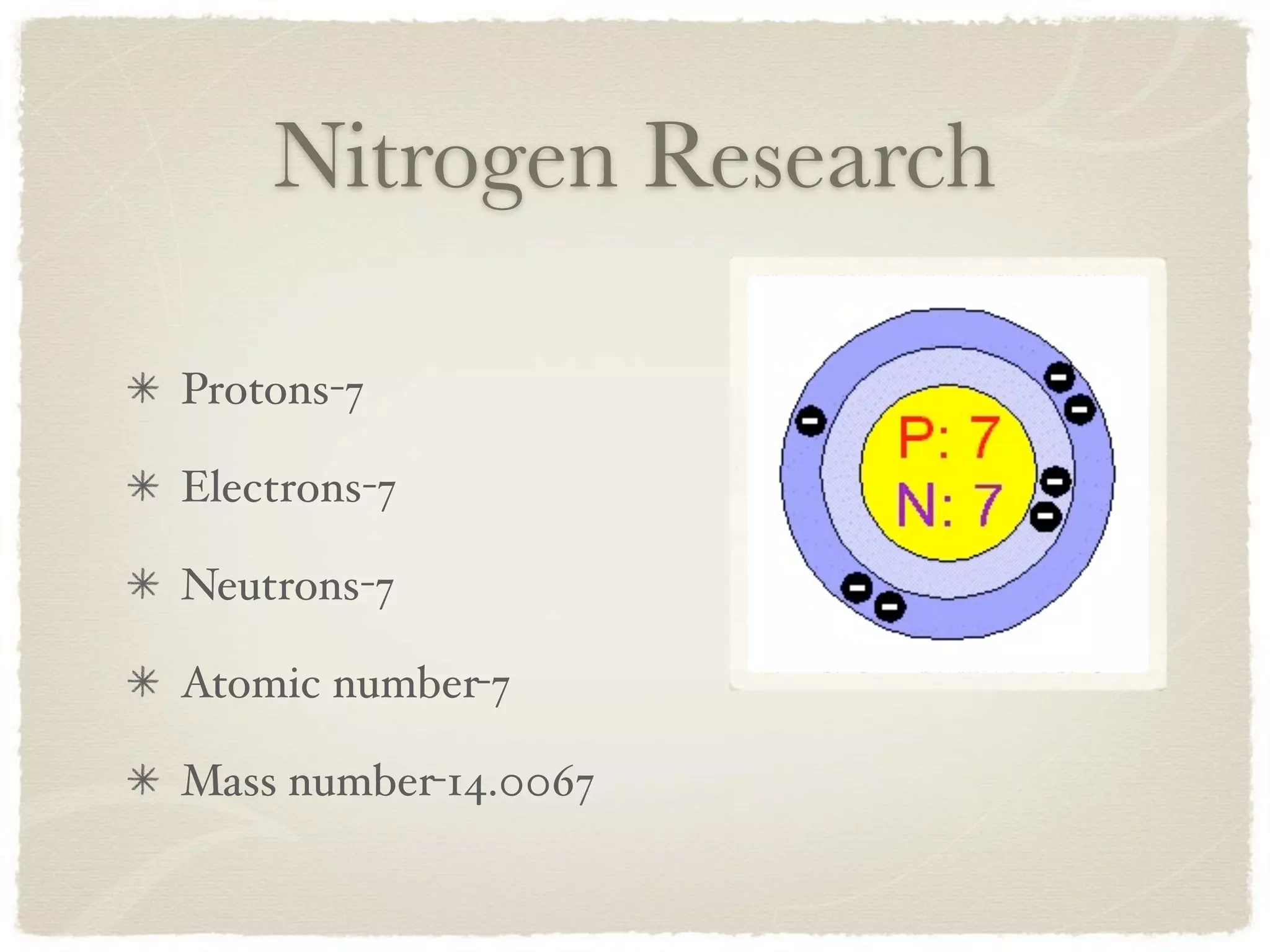
Nitrogen is a colorless, odorless non-metal gas that was discovered in 1772 by Daniel Rutherford. It has 7 protons, 7 electrons, 7 neutrons, and an atomic number of 7. Nitrogen is used in many common products like lightbulbs, refrigeration devices, and in manufacturing stainless steel. It is also used as a power source for paintball guns.