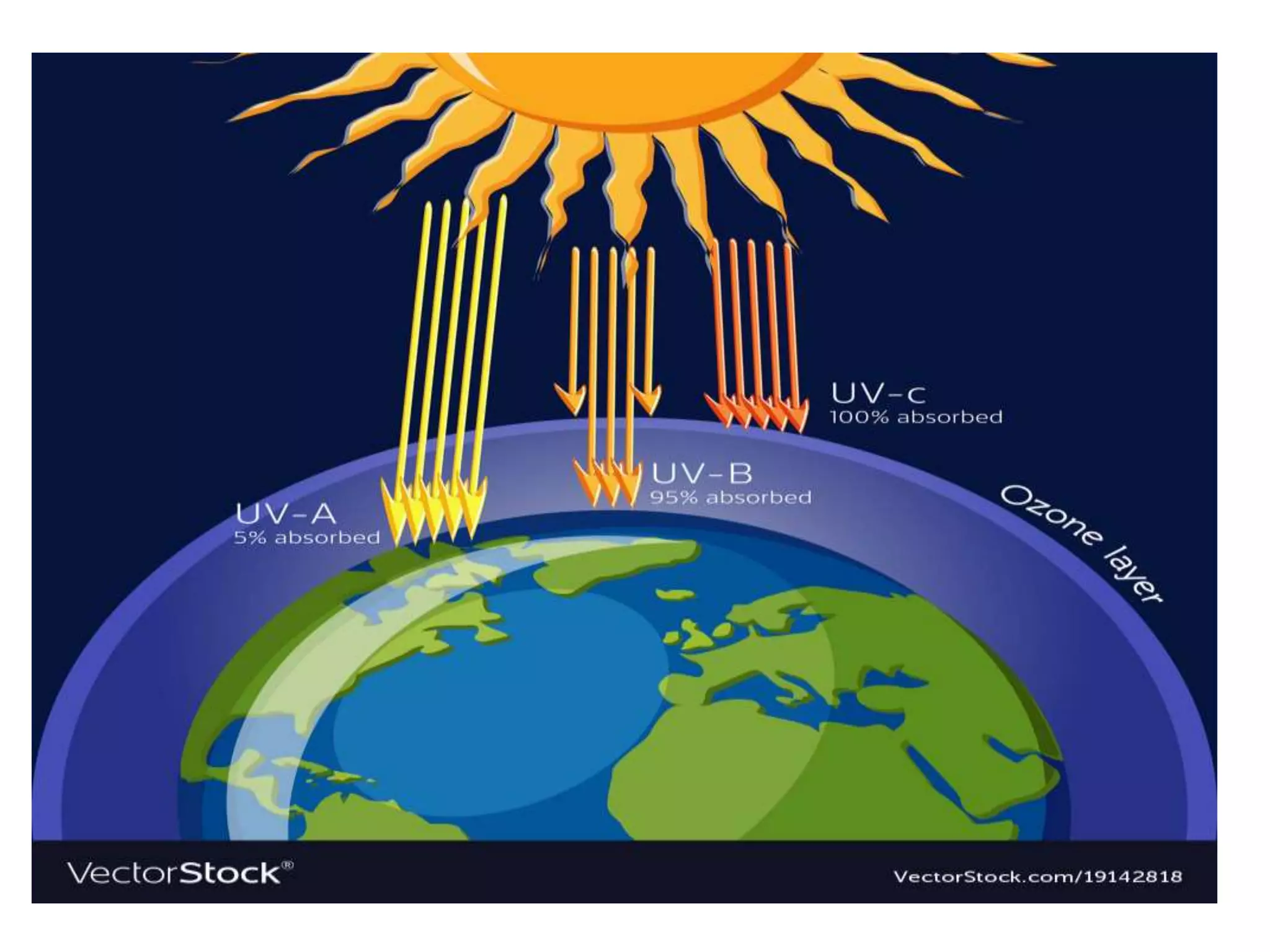
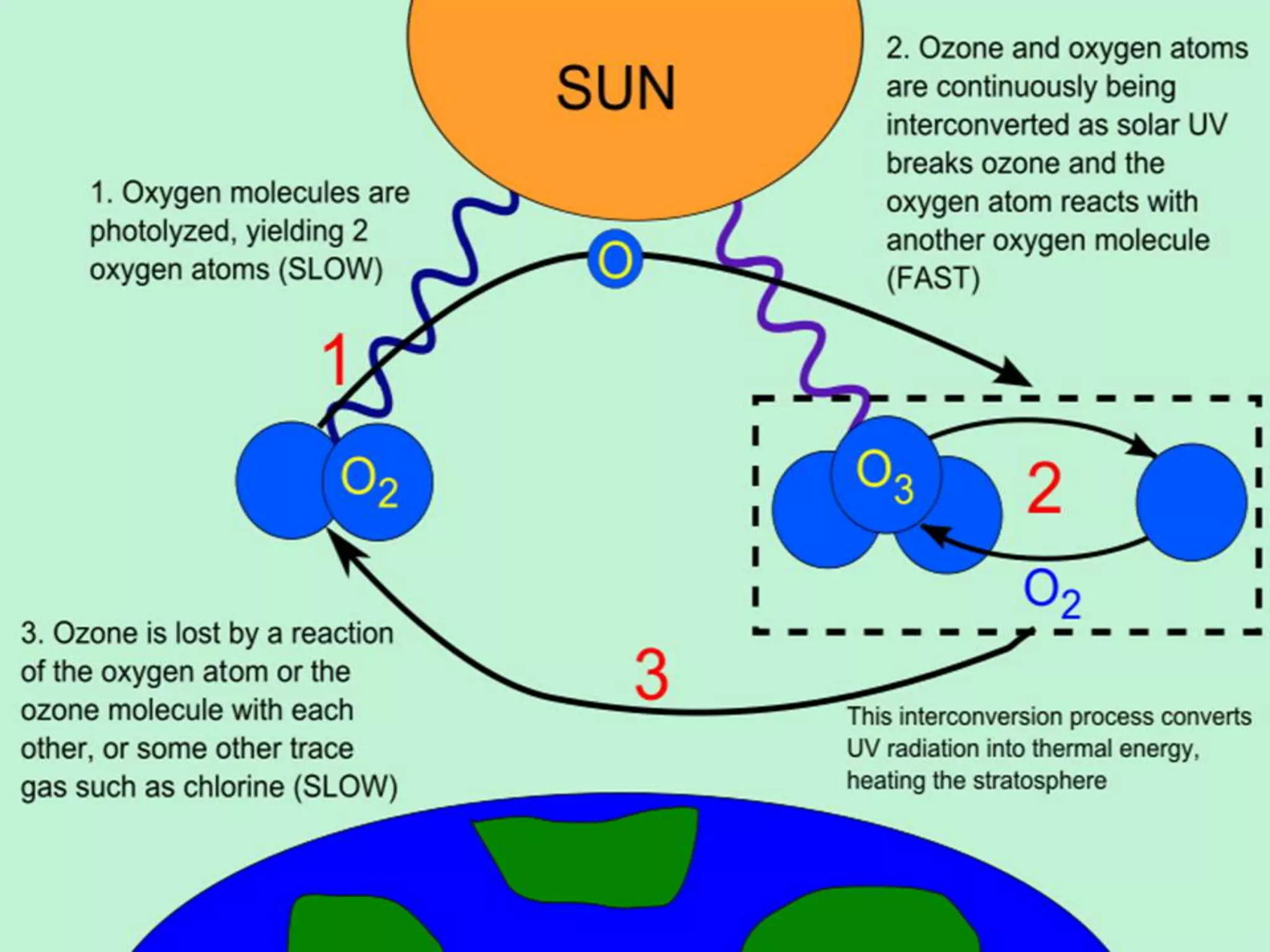
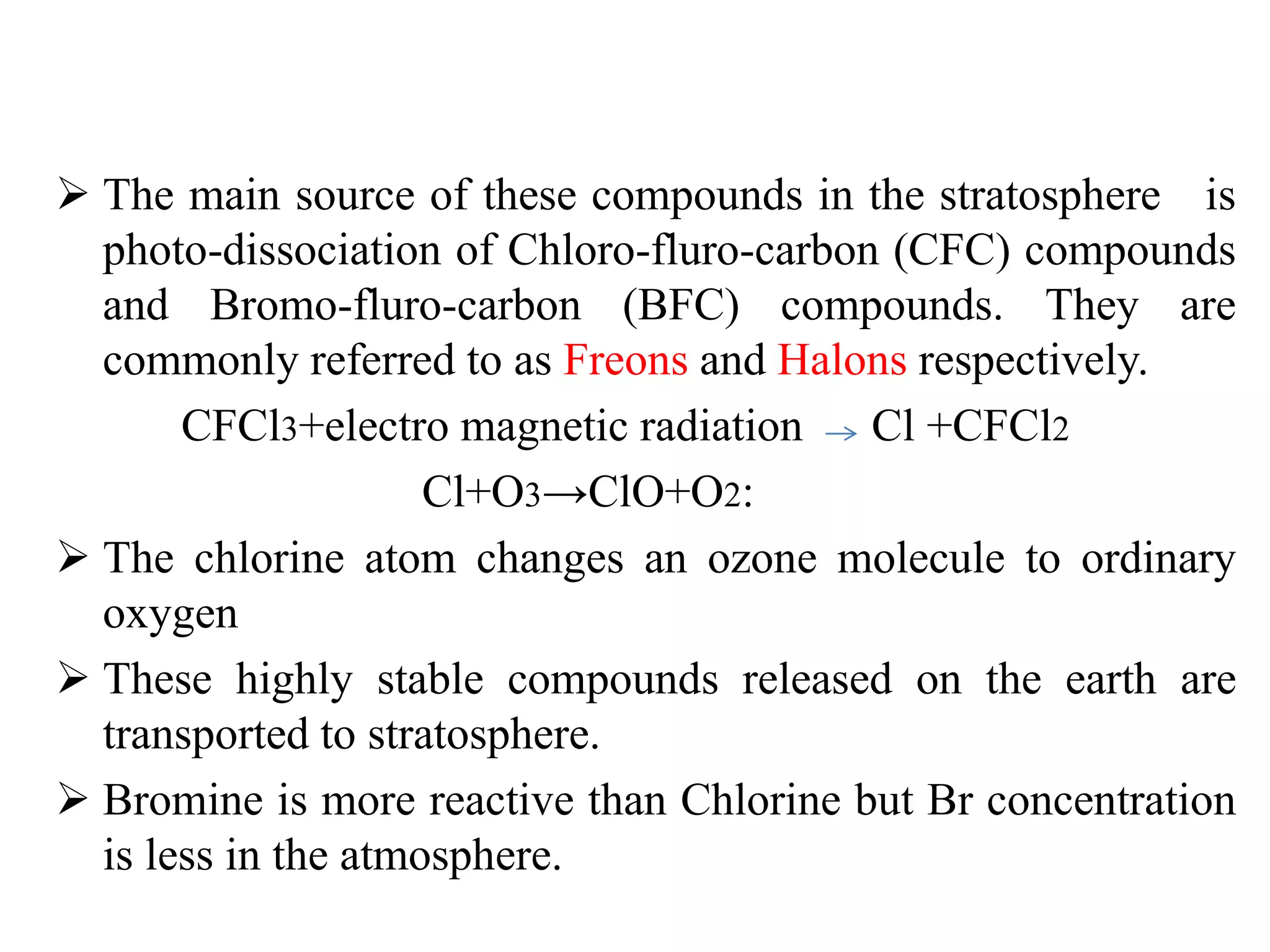
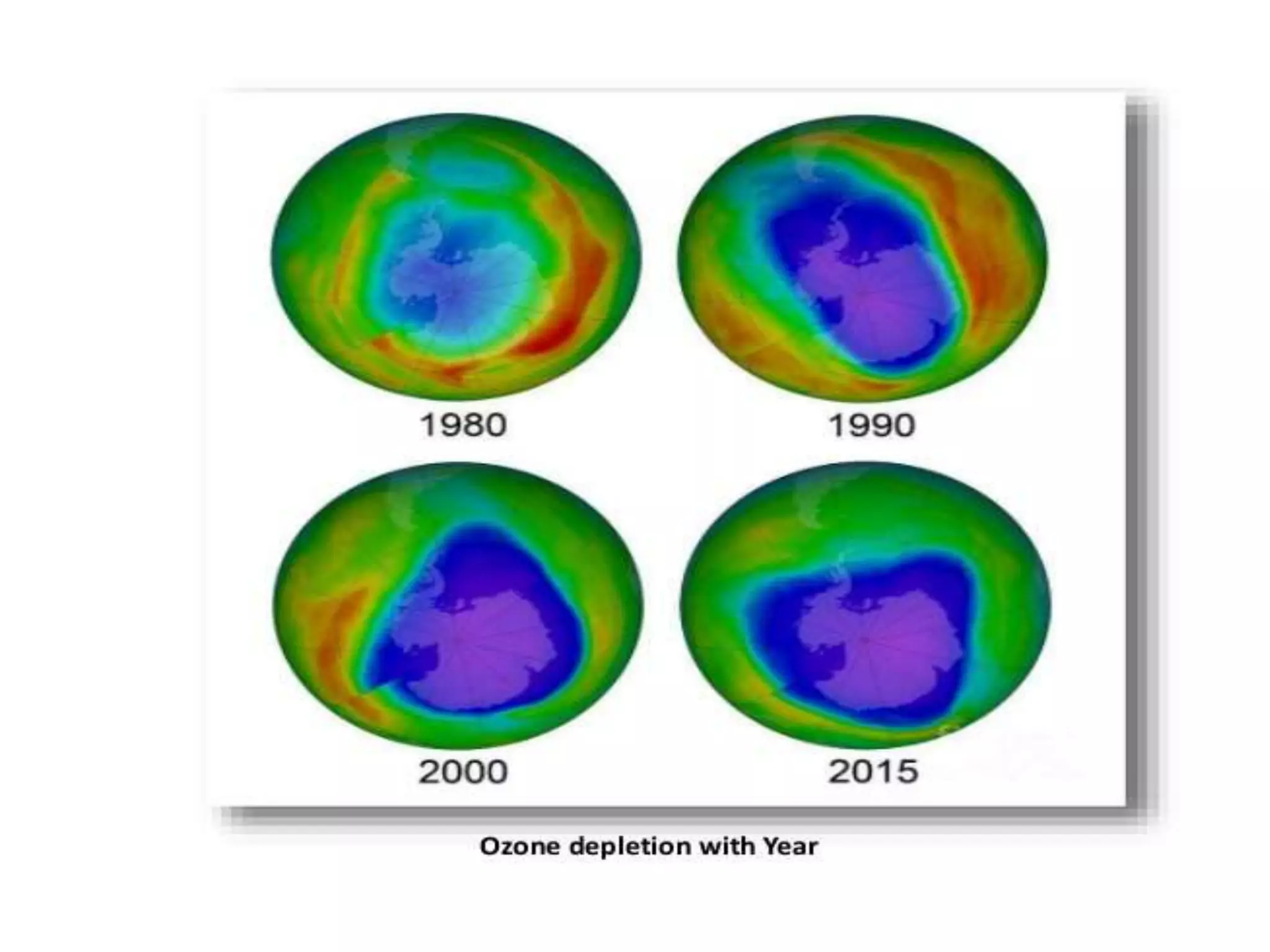
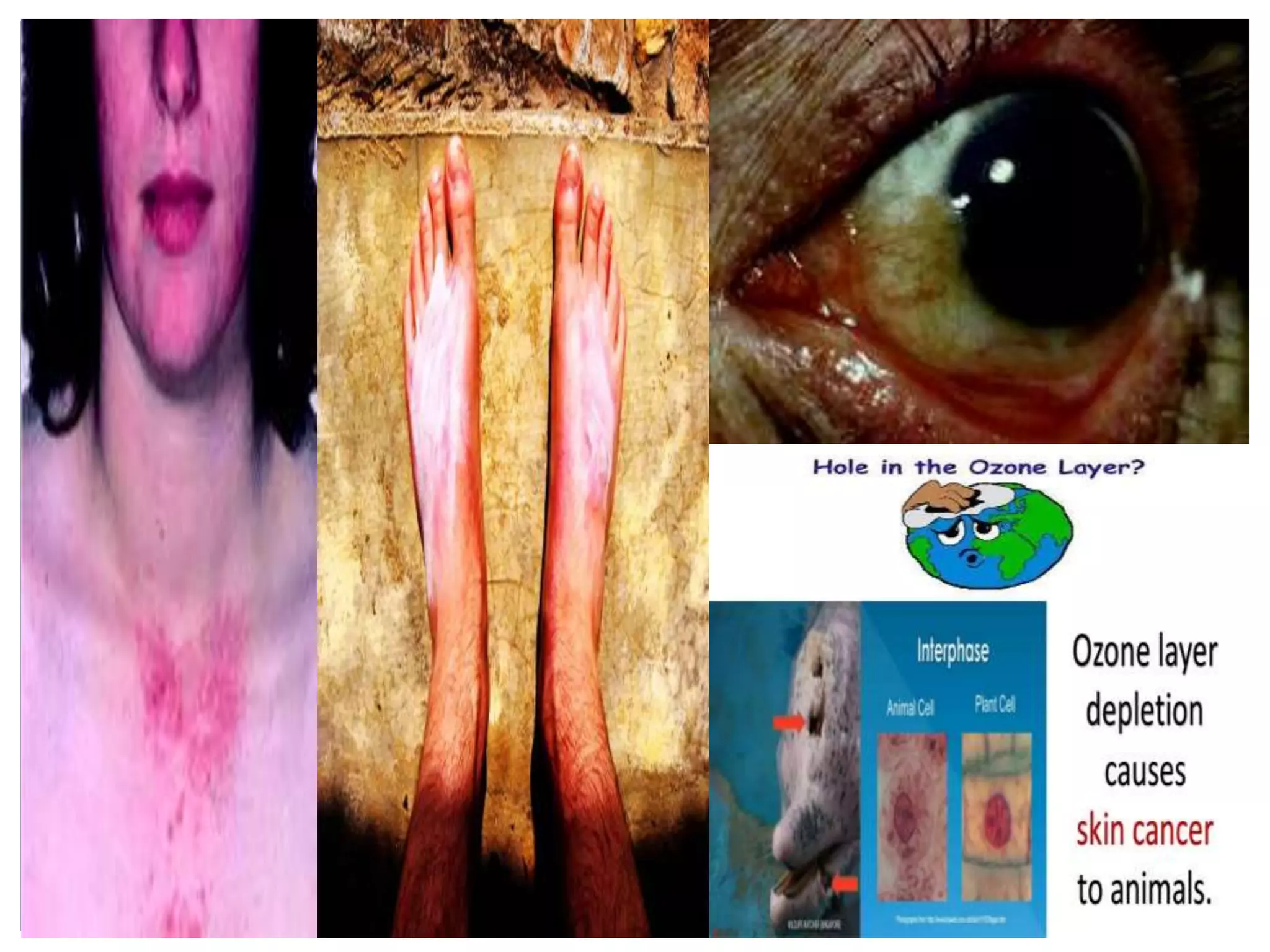
The document discusses the ozone layer, its importance in protecting life from UV radiation, and threats to its integrity. It notes that the ozone layer is found in the stratosphere and filters out much of the sun's harmful UV rays. It then explains how CFCs and other ozone depleting substances released chemicals that break down the ozone layer when they reach the stratosphere. The largest ozone hole is observed annually over Antarctica in spring due to chemical reactions on polar stratospheric clouds. The Montreal Protocol was enacted to phase out ozone depleting substances and has led to signs of recovery in the ozone layer.