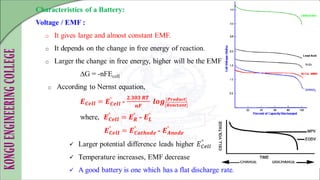
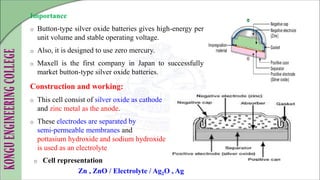
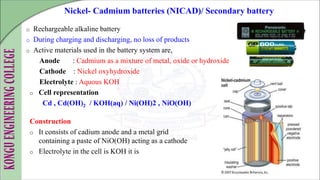
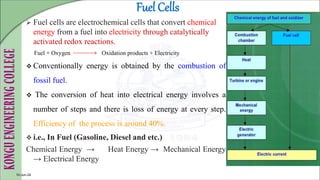
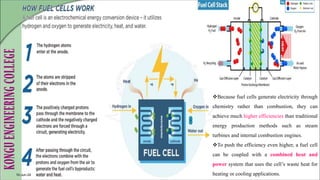
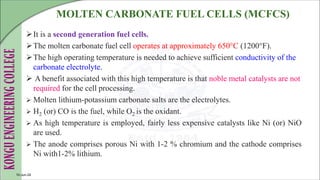
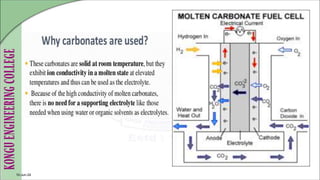
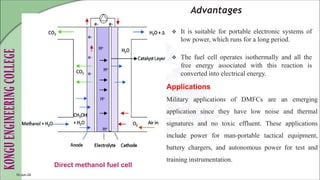
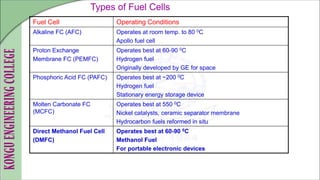
The document discusses electrochemical storage devices, focusing on various types of batteries including primary (non-rechargeable) and secondary (rechargeable) batteries, their characteristics, charging, and discharging processes. It also covers fuel cells, detailing their construction, principles, classifications, and applications, highlighting their efficiency compared to conventional energy production methods. The information provided aims to educate on the functionality, advantages, and disadvantages of different battery types and fuel cells.