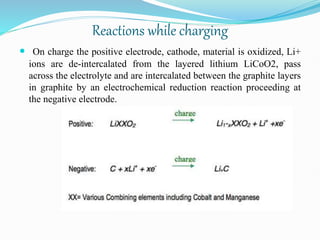
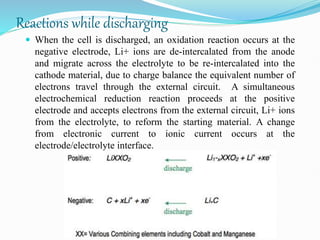
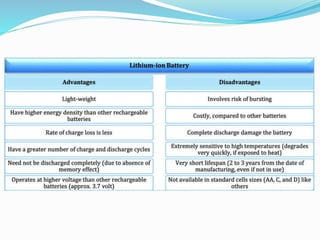
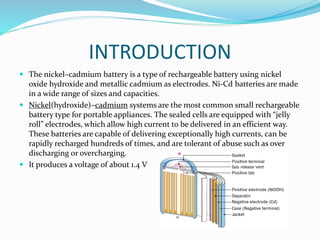
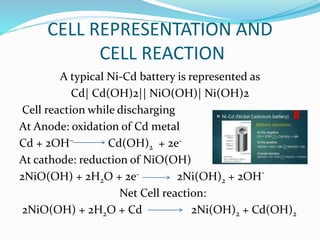
The nickel-cadmium battery uses nickel oxide hydroxide and cadmium as electrodes. It was invented in 1899 and further improved to be sealed and absorb gases generated during charging. Ni-Cd batteries were commonly used in power tools, radios, and cameras due to their high current delivery and rapid recharging ability. However, concerns over cadmium toxicity and the development of newer batteries like nickel-metal hydride and lithium-ion that are cheaper and less toxic have reduced Ni-Cd battery usage.









![Chemical Reactions
Main essential components...
Anode: Graphite [carbon] - C(s)
Cathode: Lithium Cobalt Oxide - LiCoO2
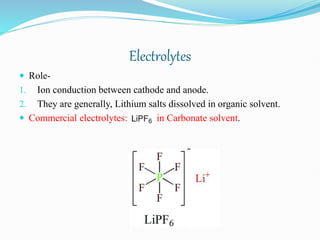
Electrolyte: Typically a combination of lithium salts - LiPF6, LiBF4,
or LiClO4, in an organic solvent, such as either.
Separator: The separator is a very thin sheet of micro perforated
plastic. - CH2=CHCl](https://image.slidesharecdn.com/battery-240326175021-9365bbbb/85/Battery-semester-1-chemistry-for-study-pptx-13-320.jpg)