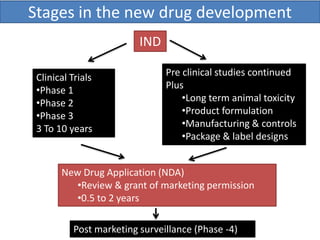
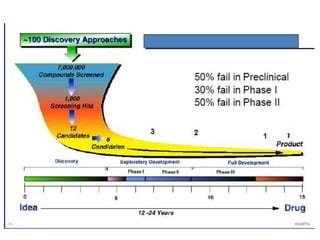
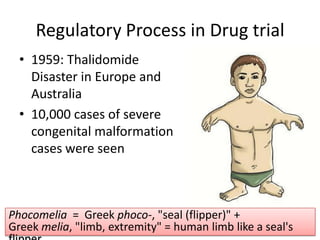
New drug development is a highly complex, costly, and time-consuming process that can take over 10 years. It involves synthesis of new chemical entities, preclinical studies in animals and cells to evaluate safety and efficacy, followed by clinical trials in humans in 4 phases to further assess safety and efficacy. If clinical trials are successful, regulatory approval must be obtained before the drug can be marketed. The overall goal is to bring new treatments to patients while ensuring safety and effectiveness through a rigorous scientific process.