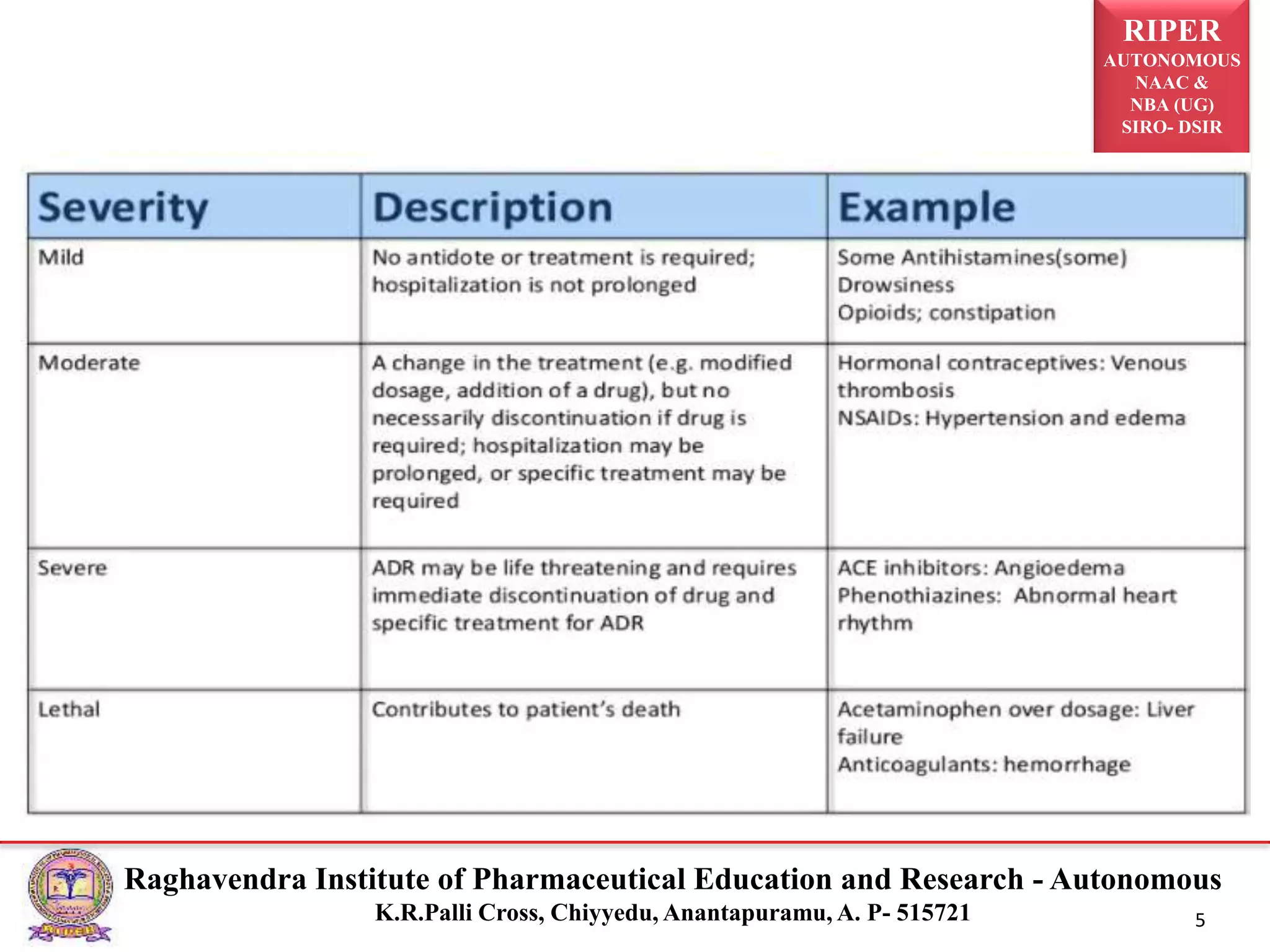
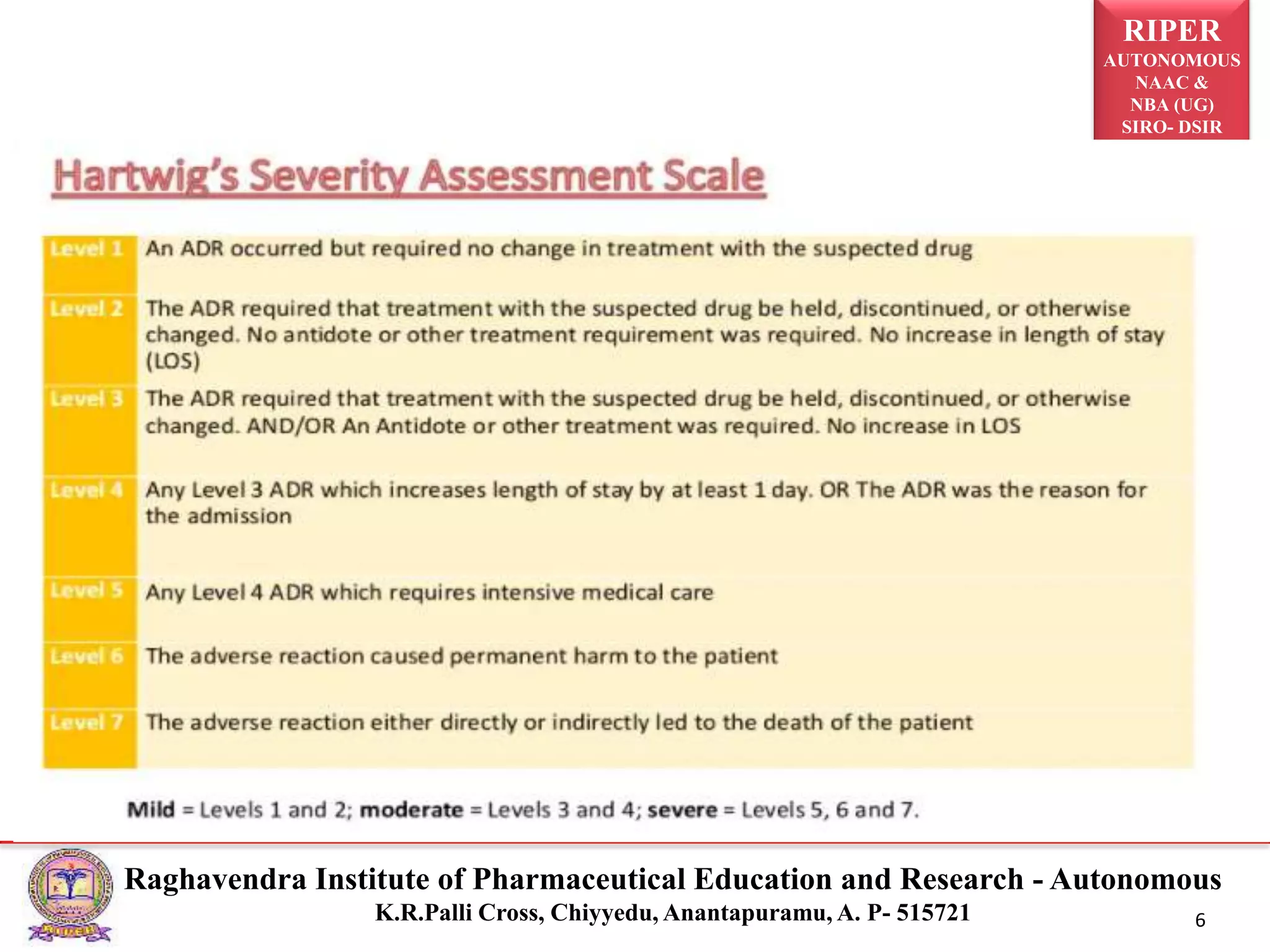
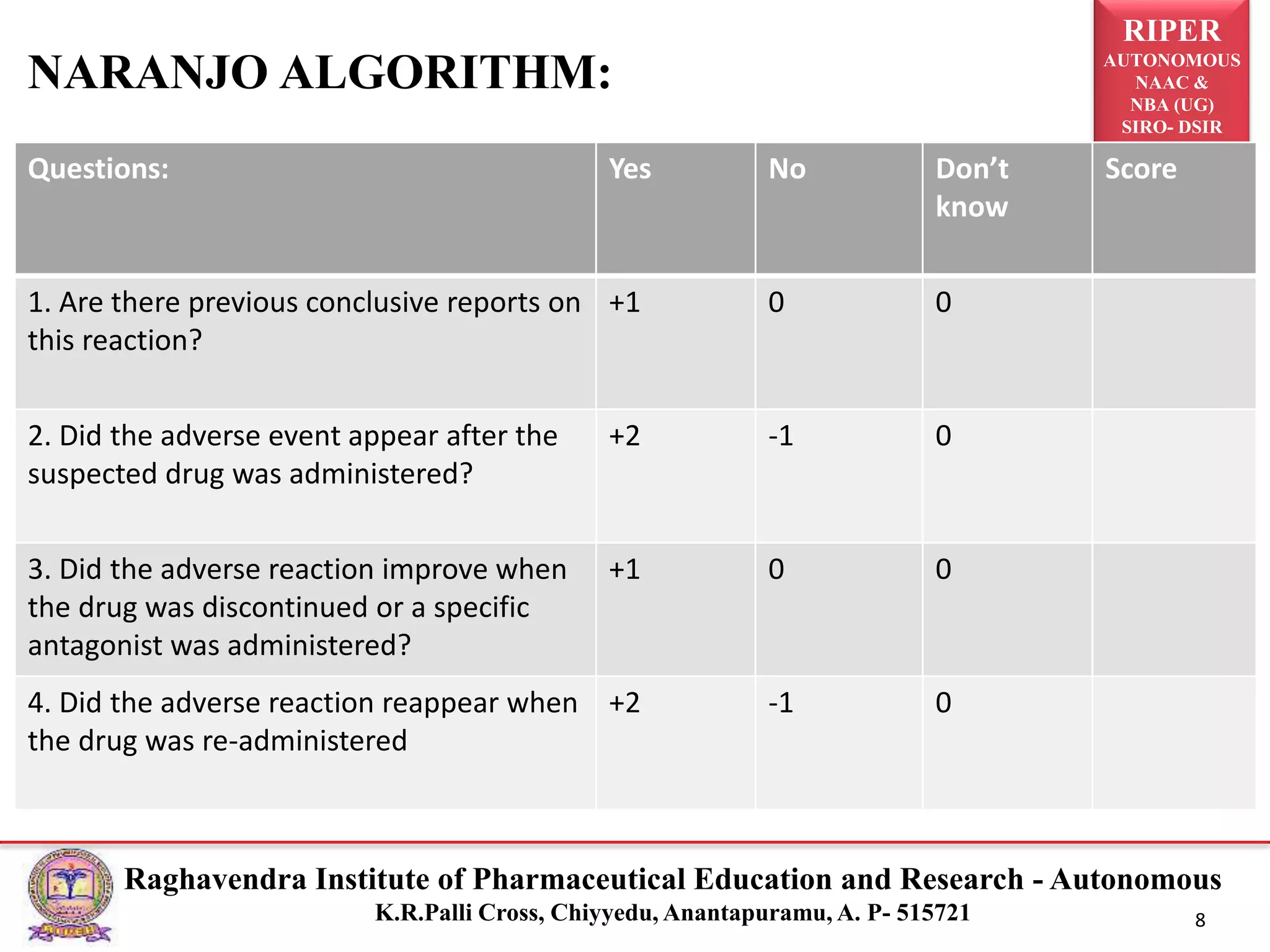
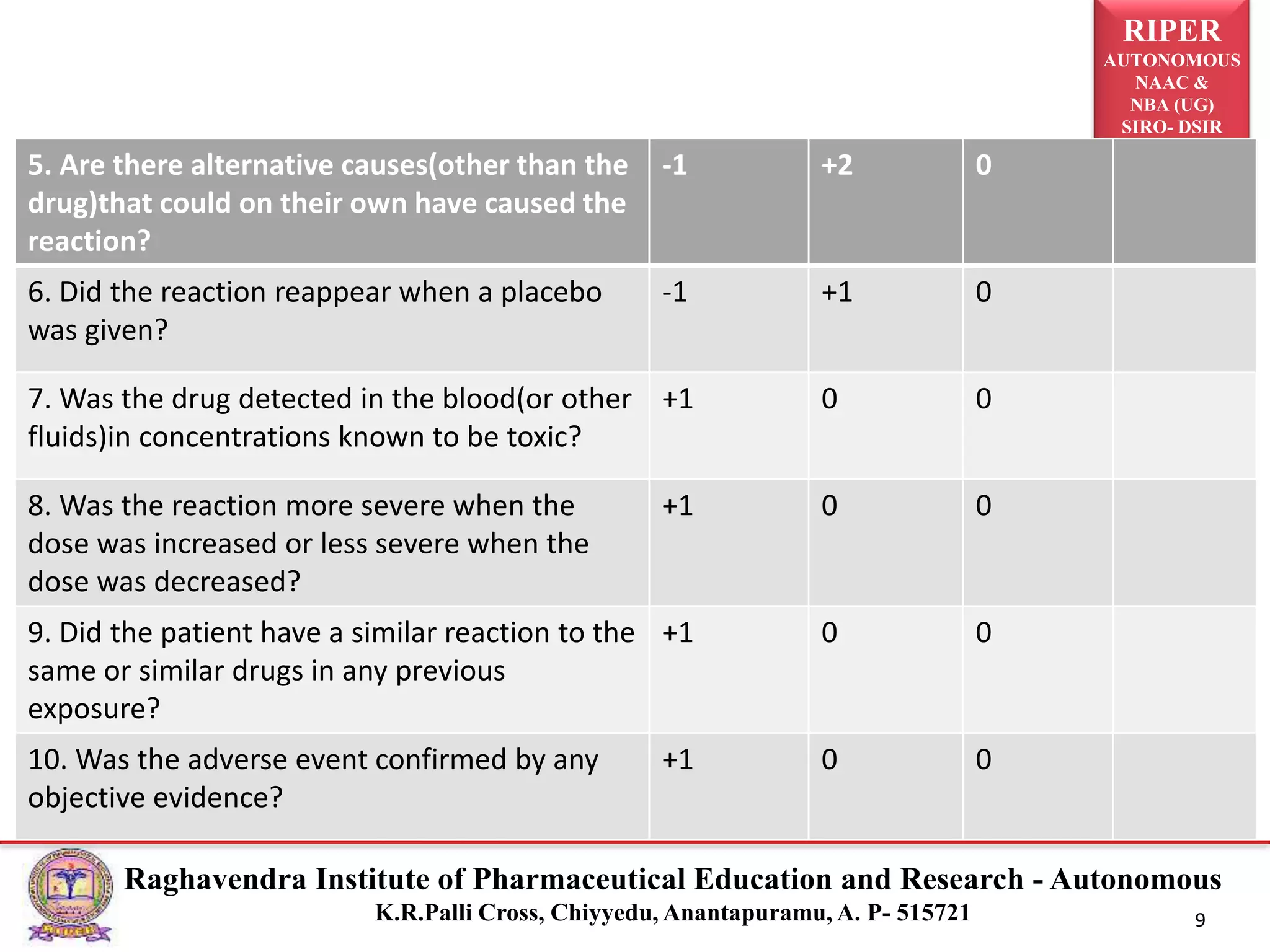
The document discusses the assessment of adverse drug reactions (ADRs), focusing on definitions, severity, seriousness, and preventability. It includes various classification systems such as Karch and Lasagna for severity and the Naranjo algorithm for determining ADR probability. Additionally, it emphasizes the significant percentage of preventable ADRs and references key literature on the topic.