Embed presentation
Downloaded 355 times





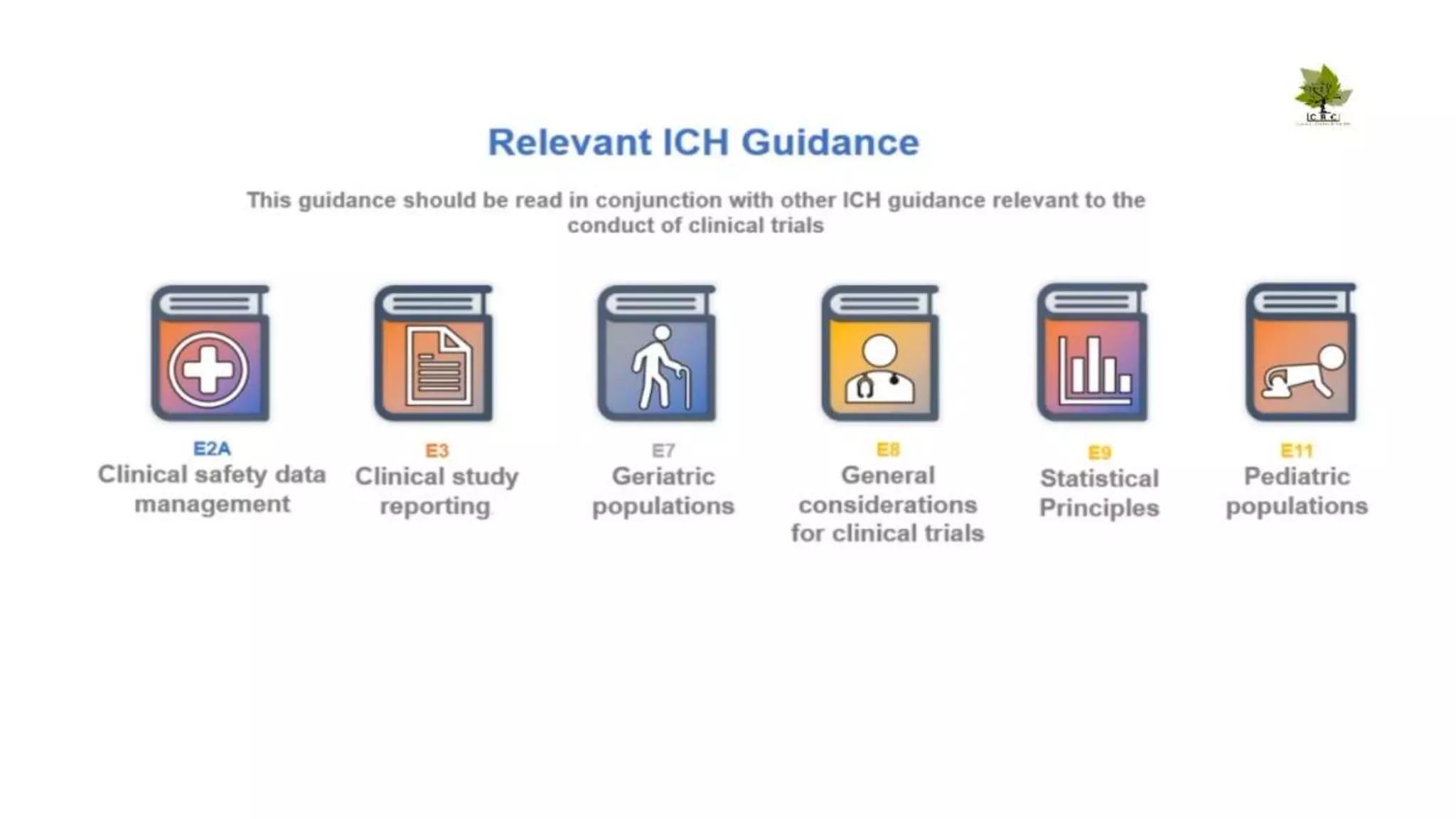









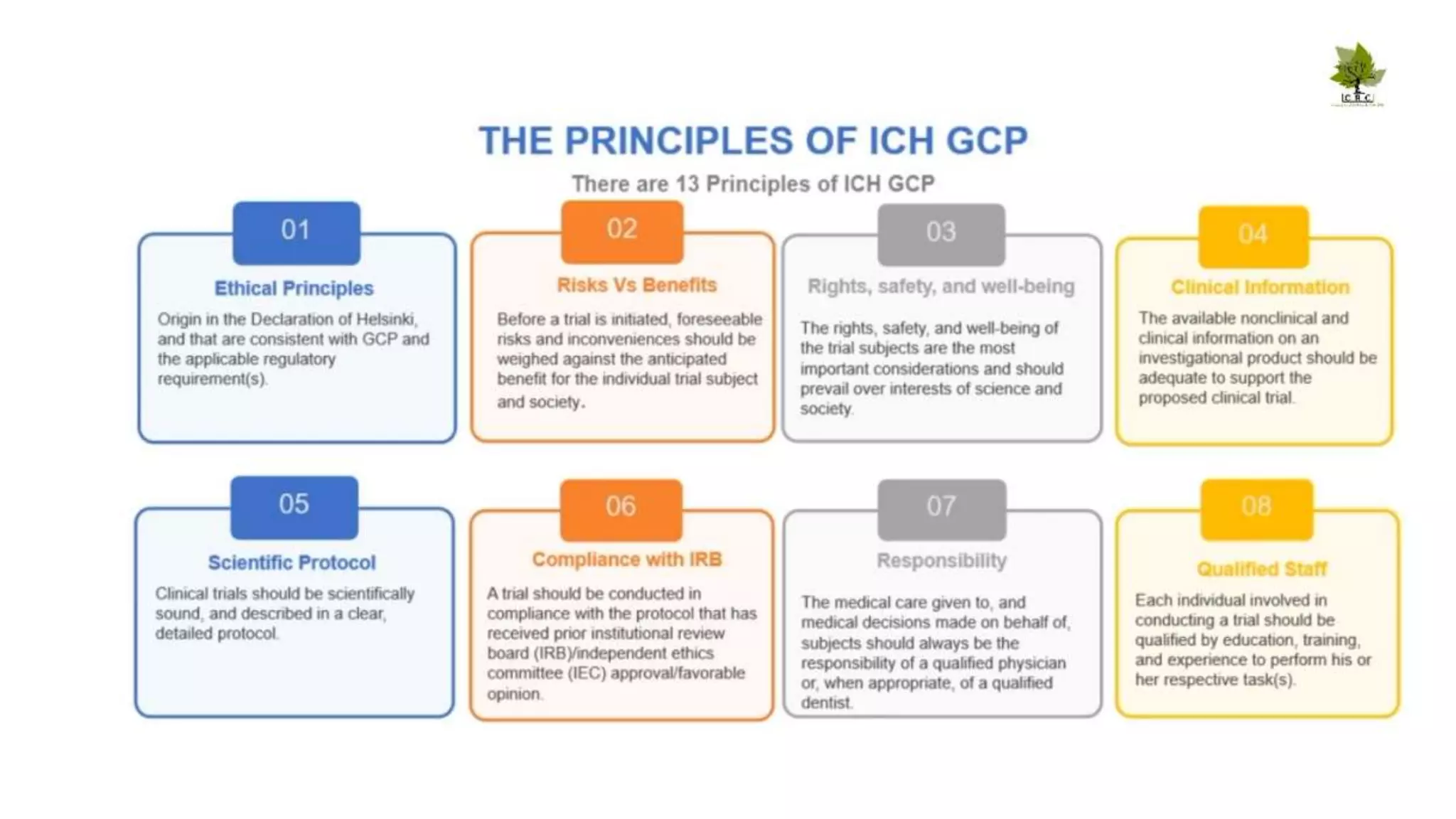
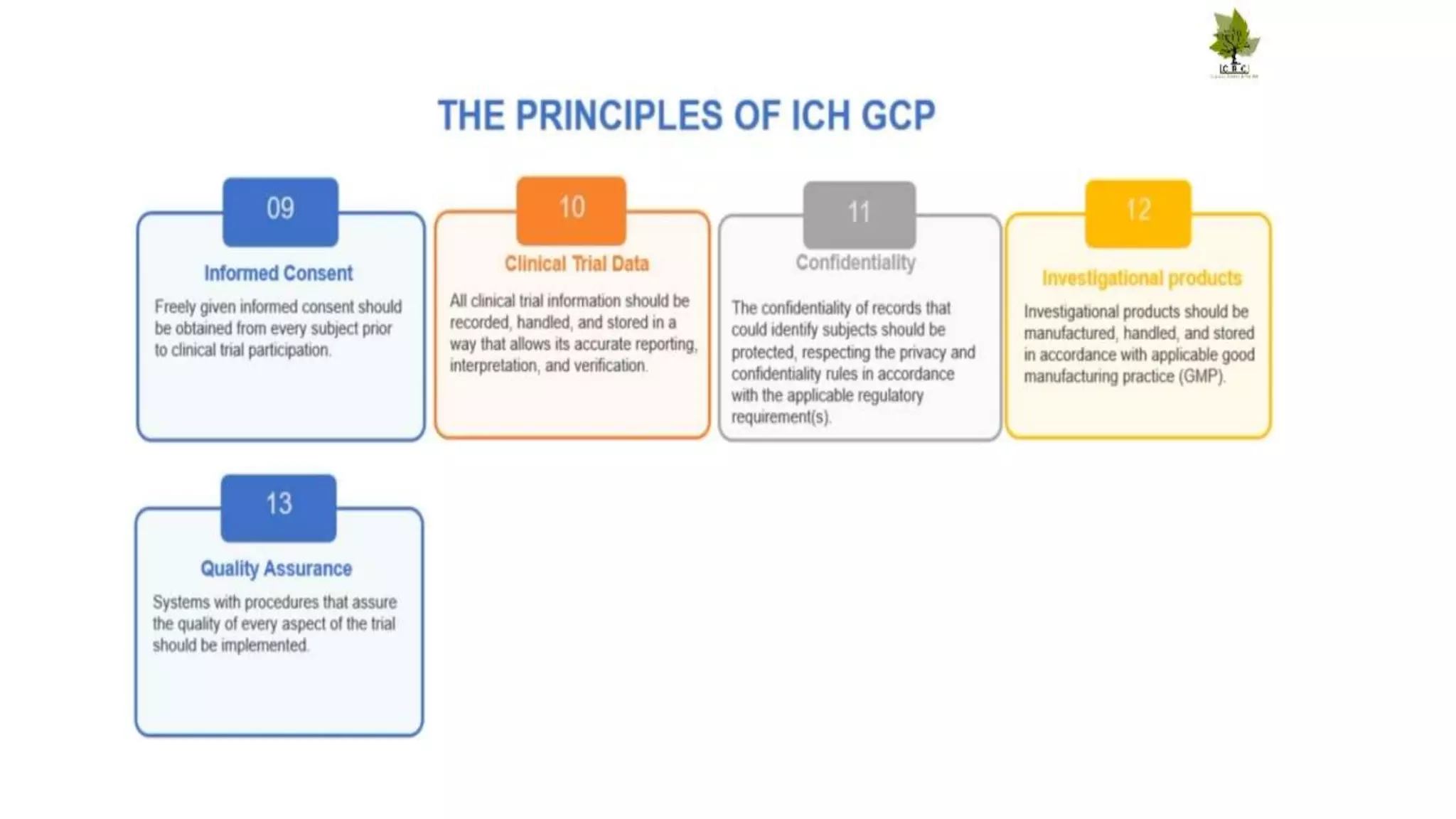

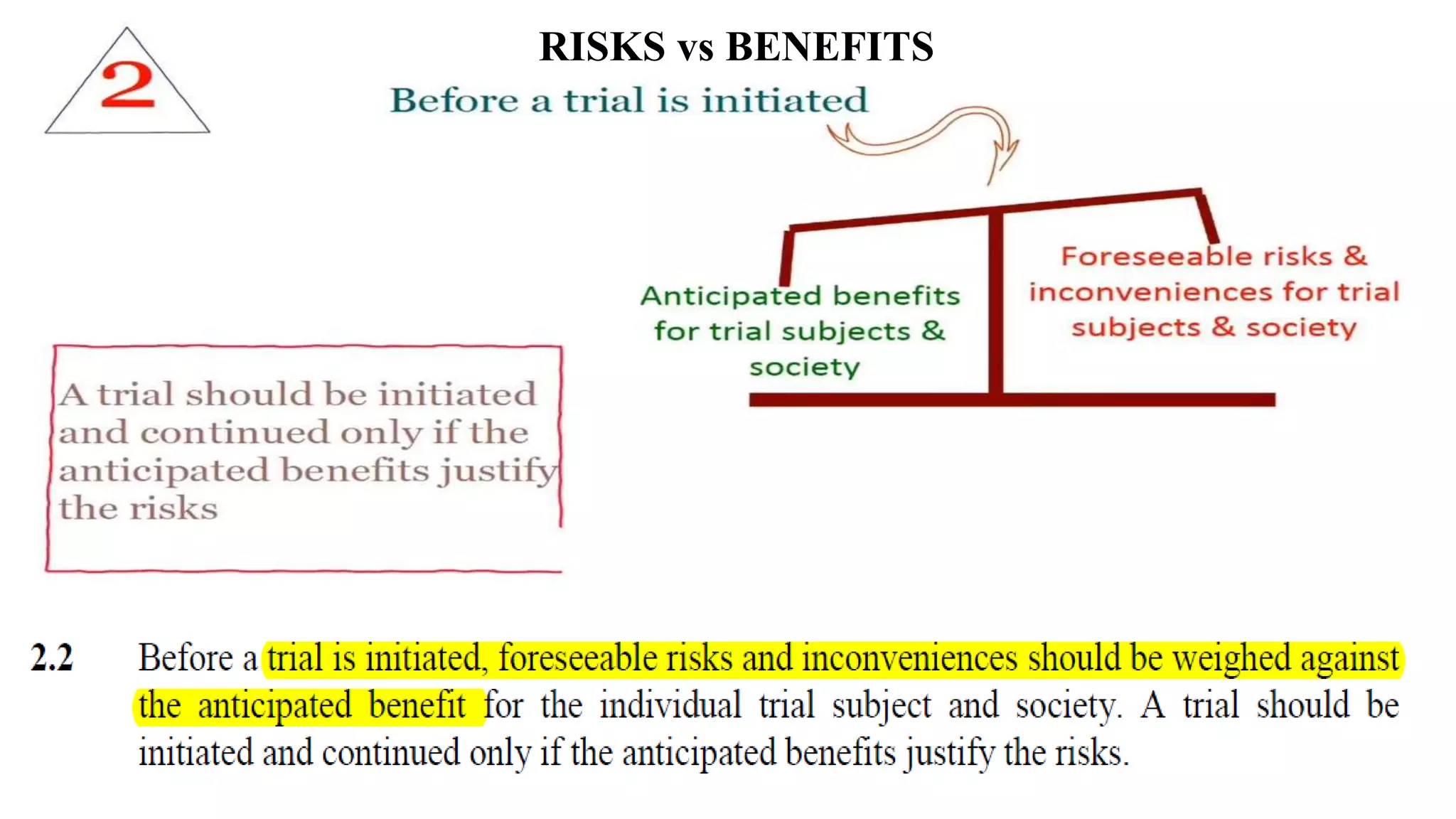

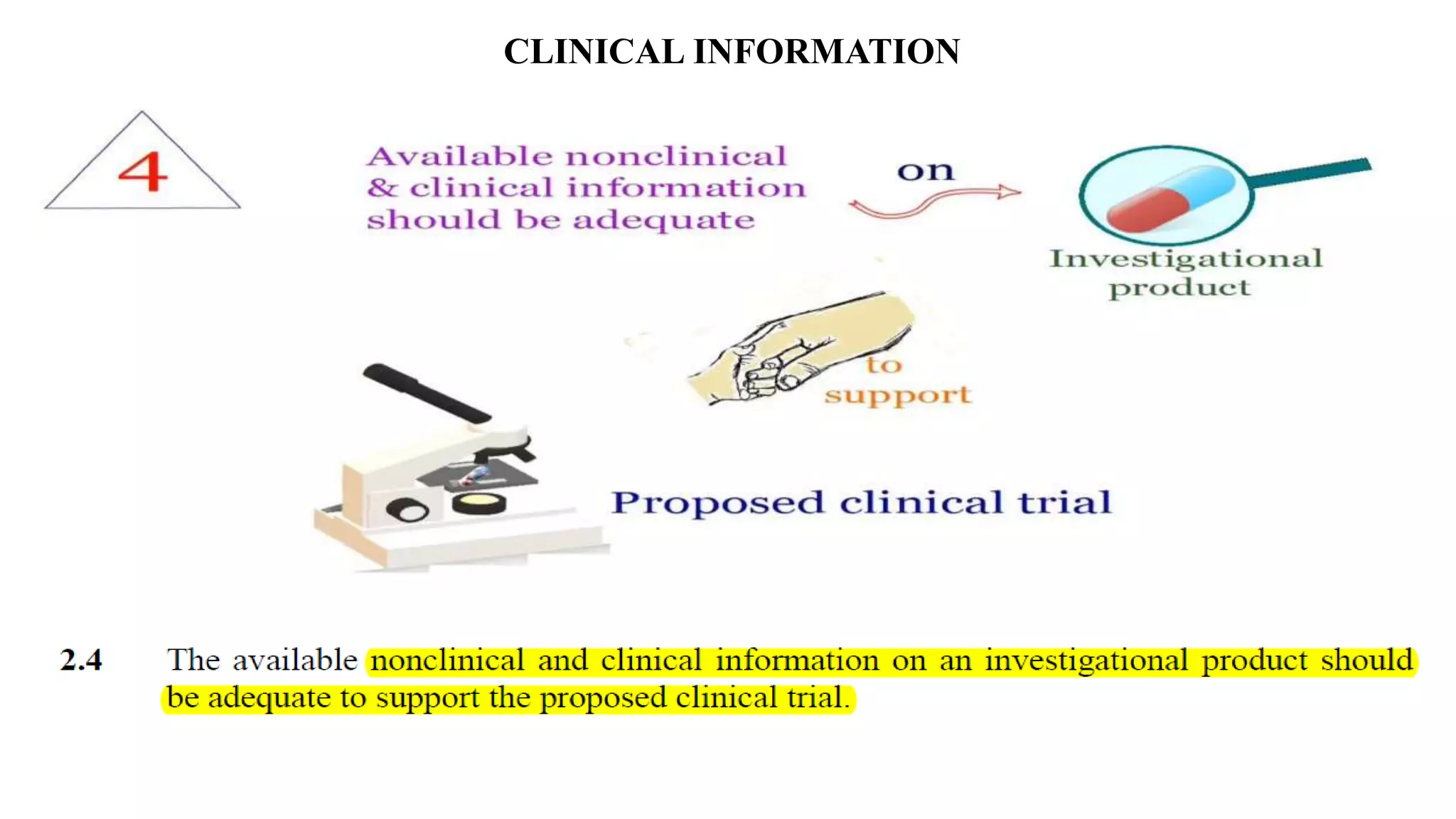














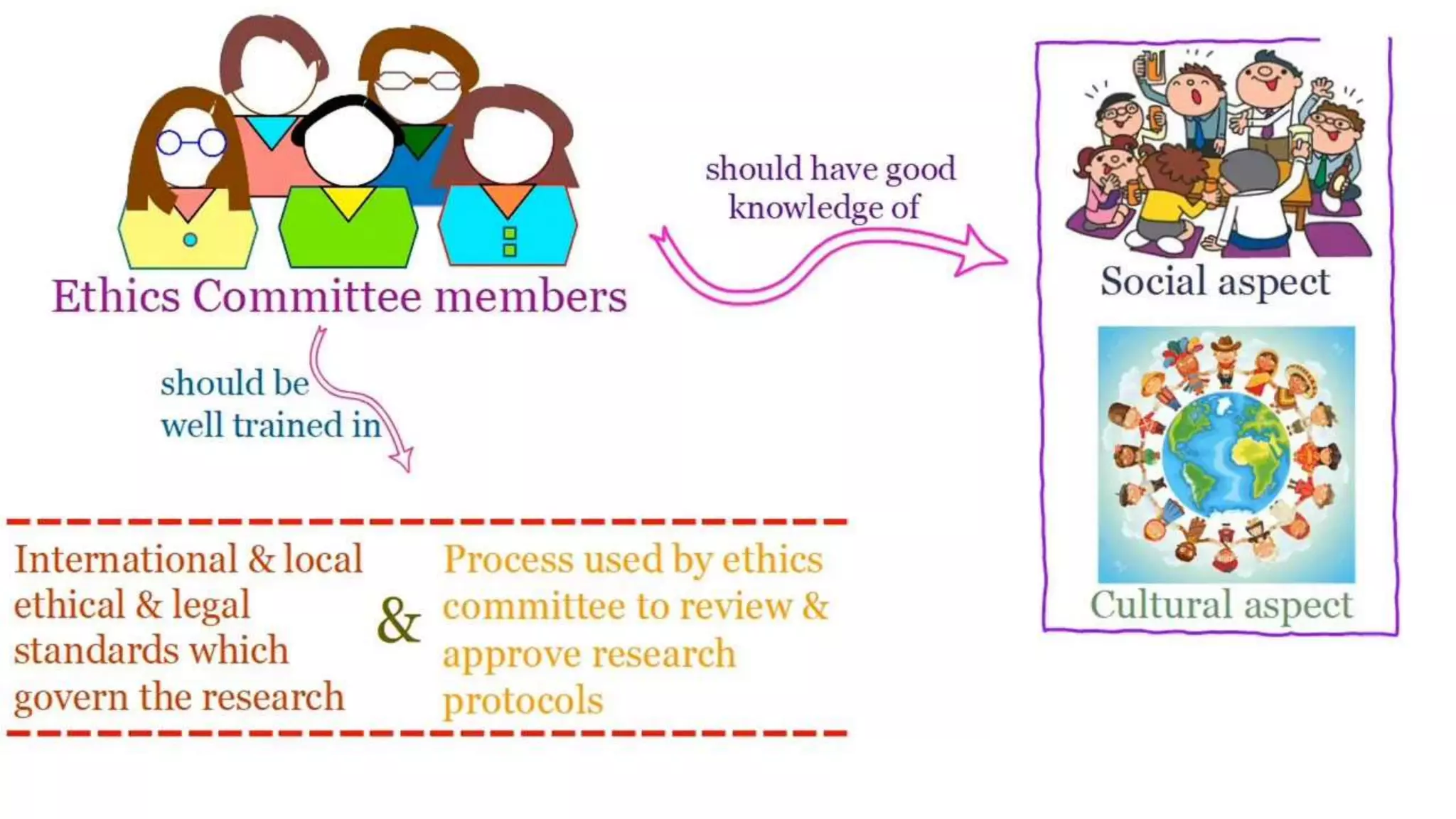

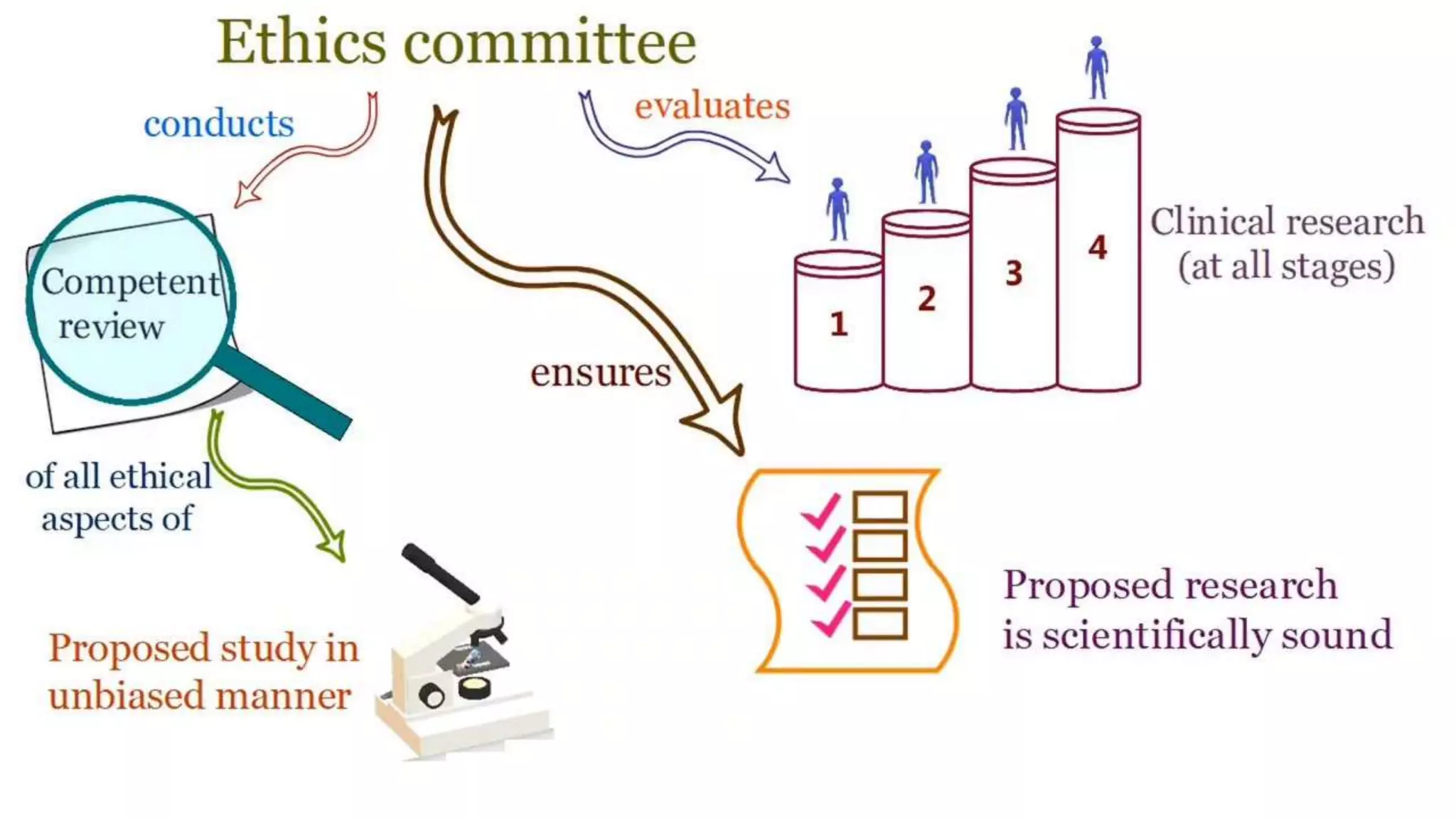






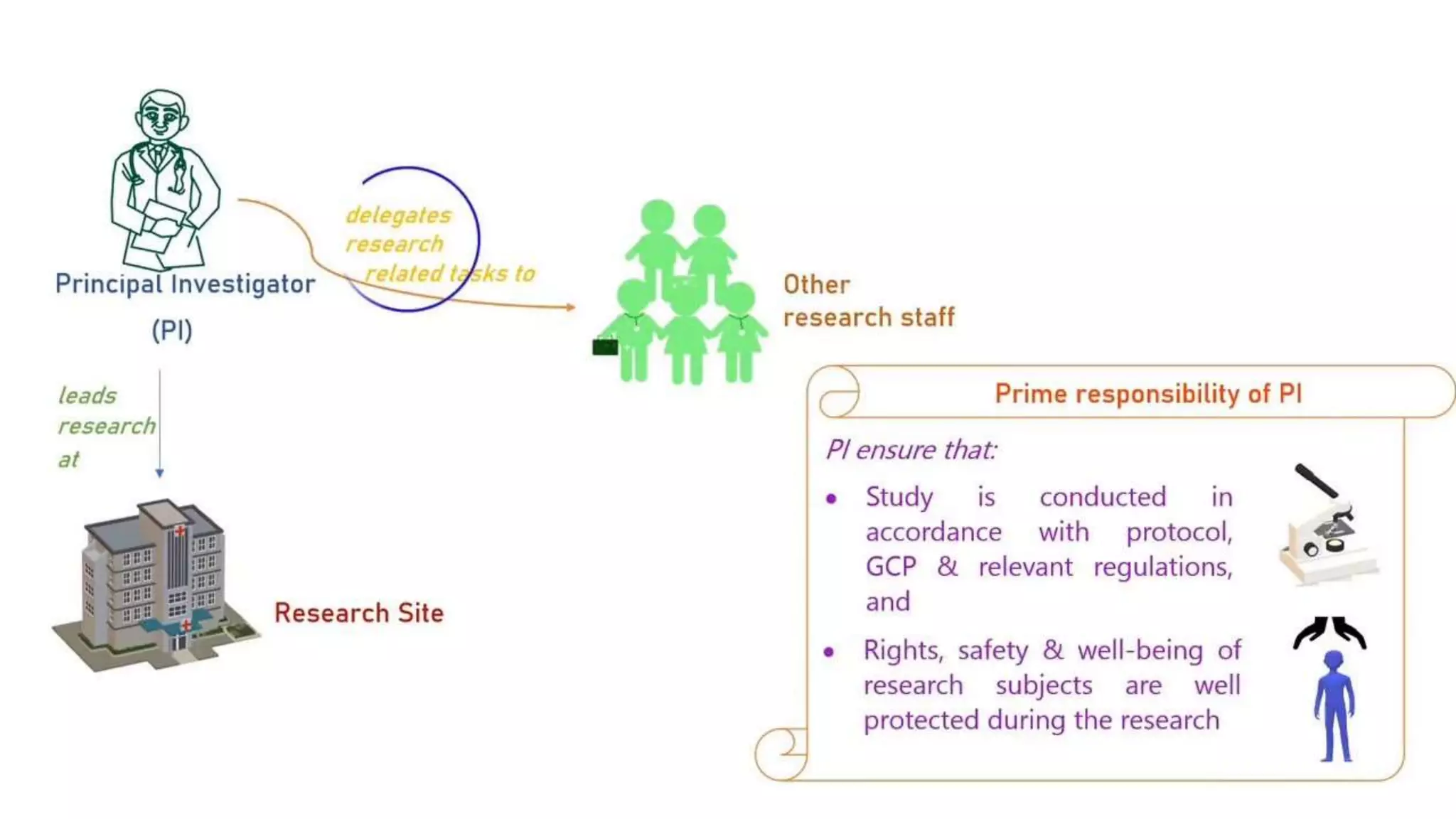


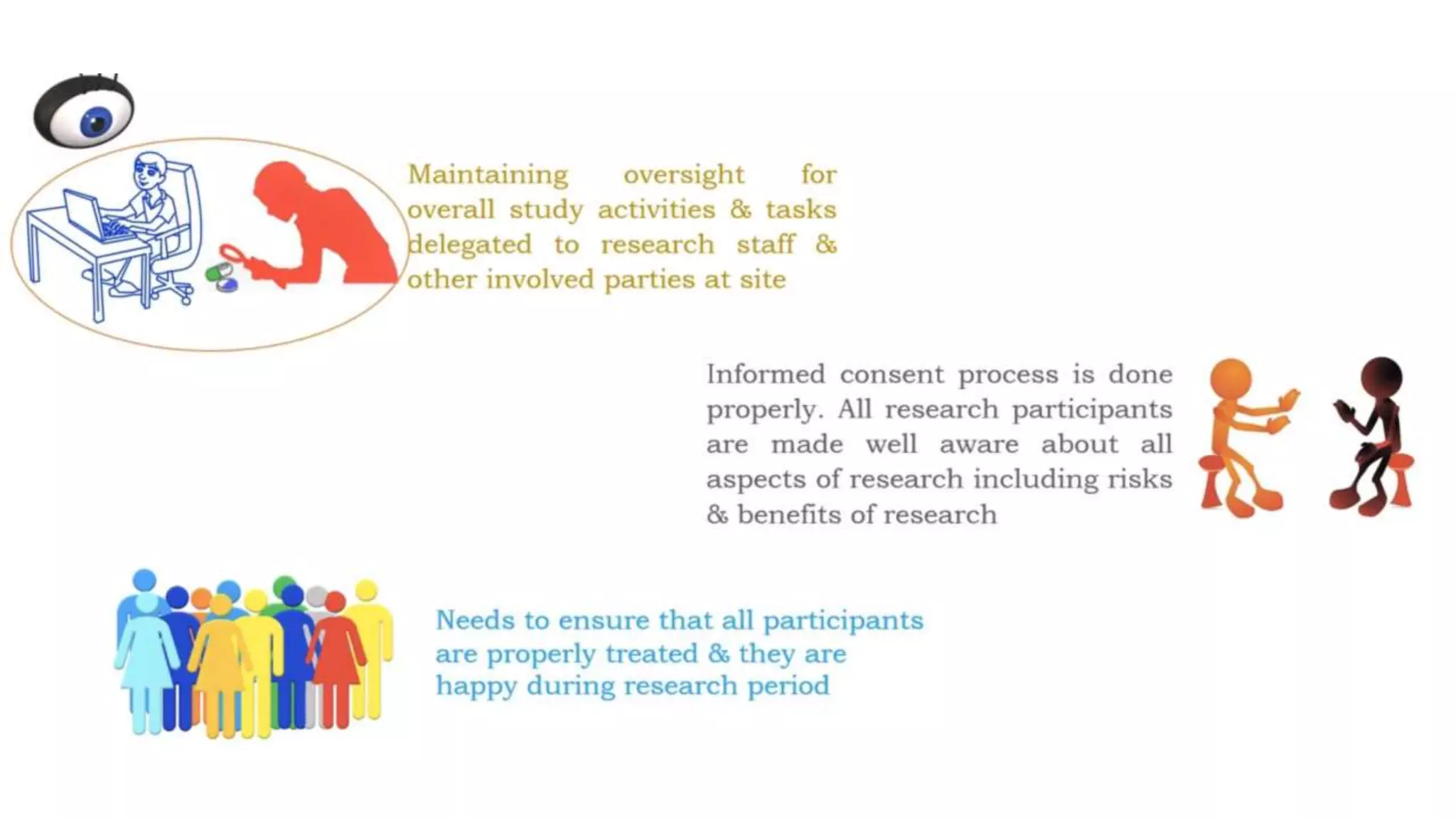











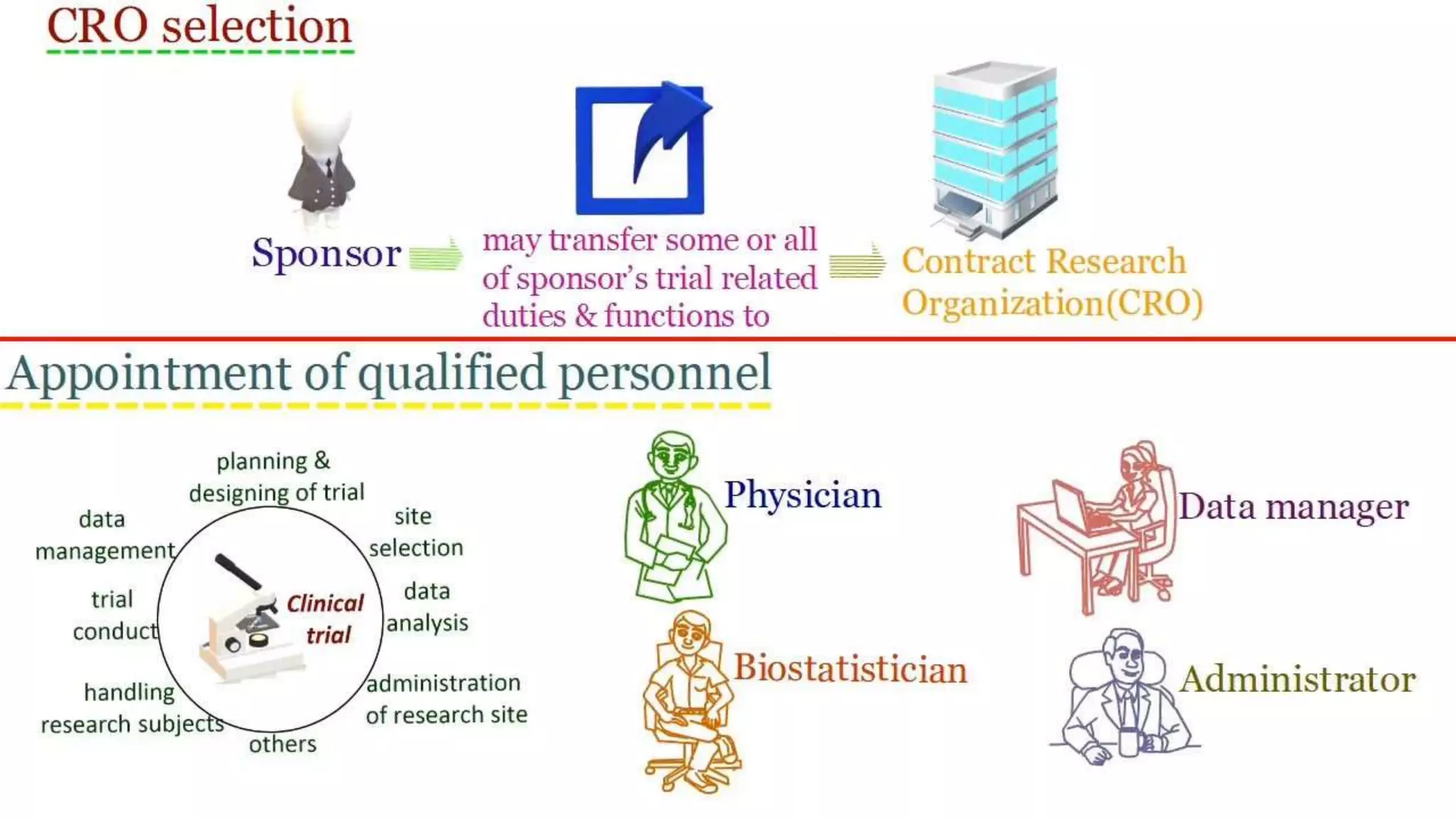

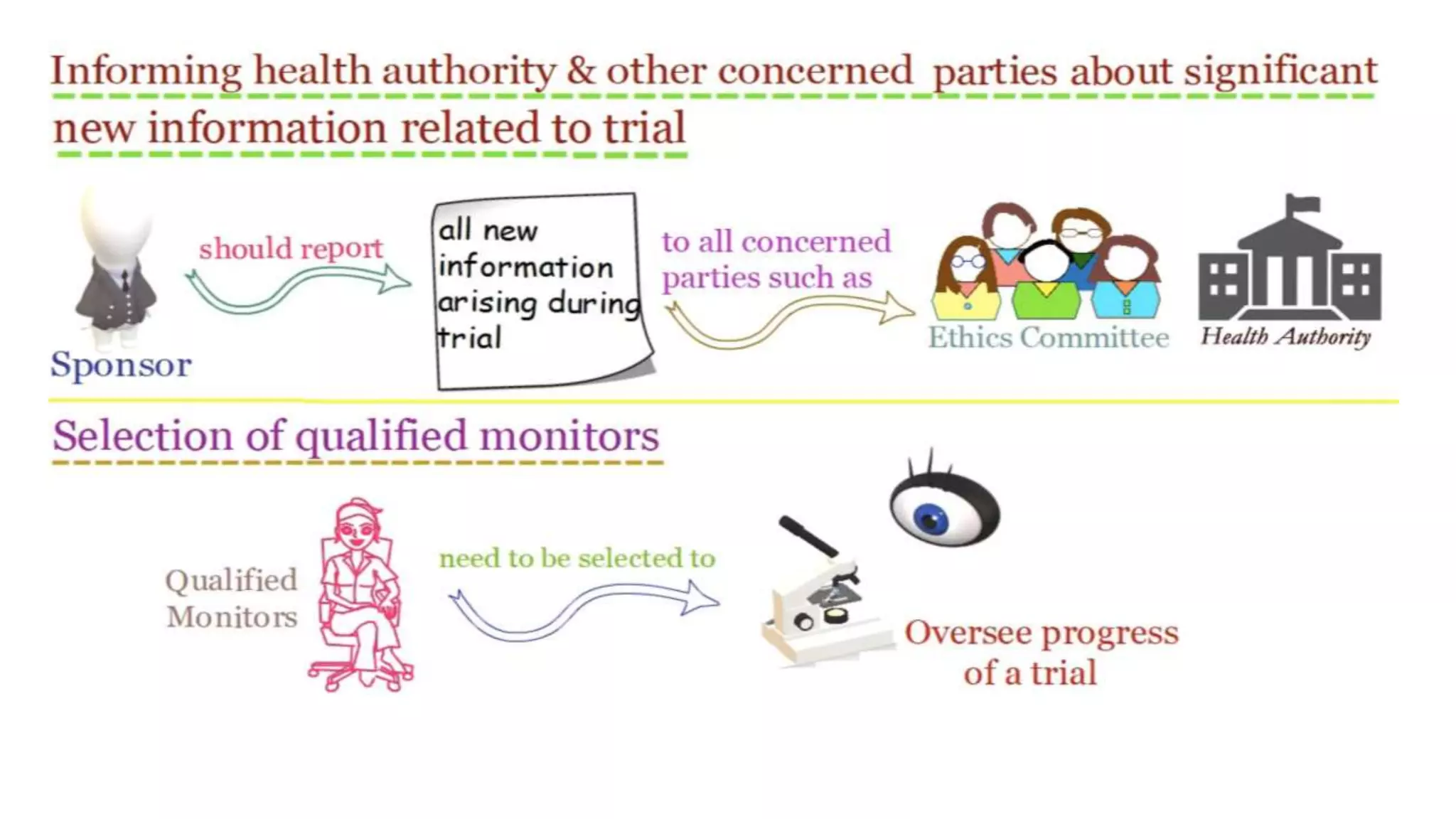



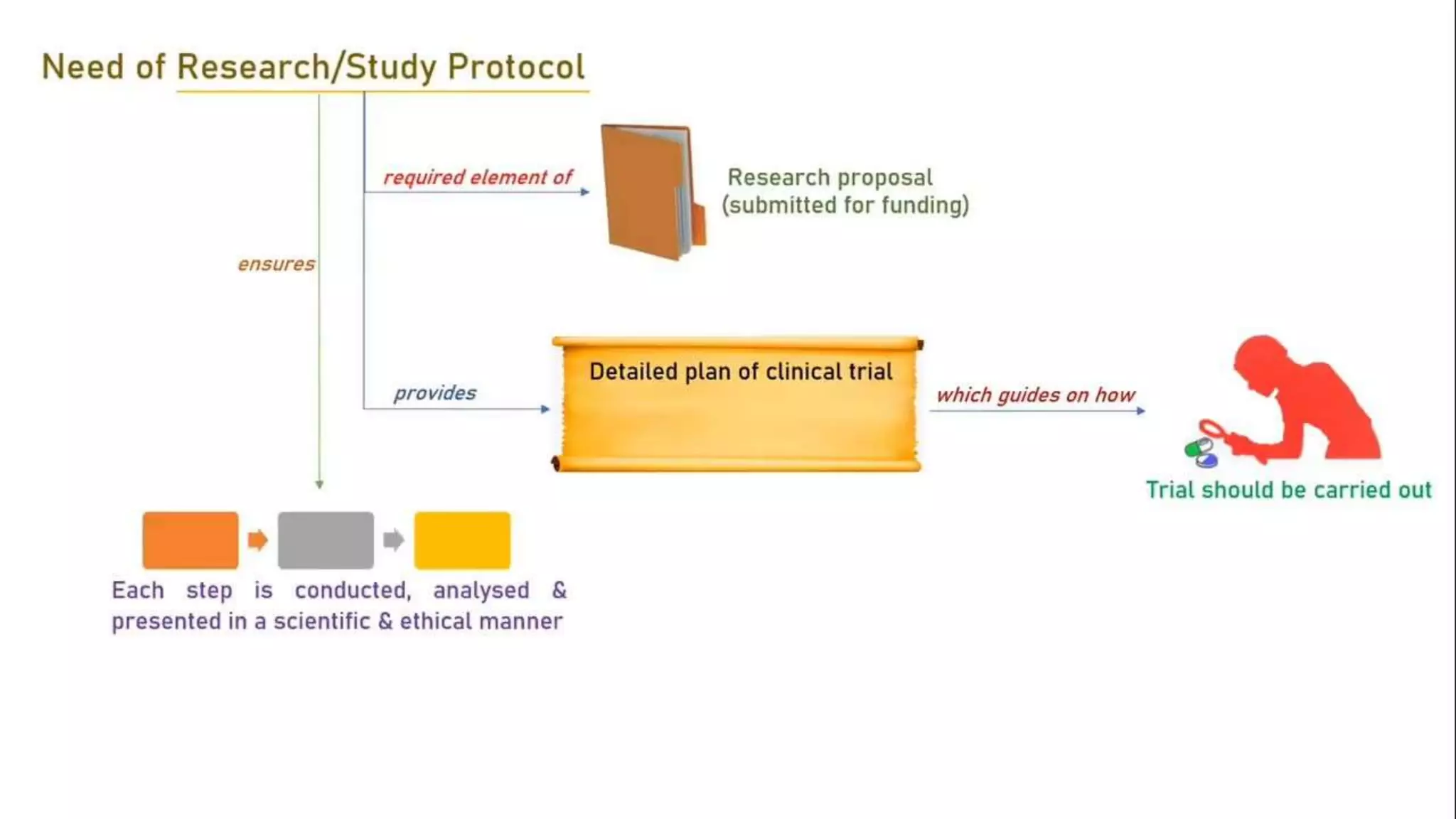
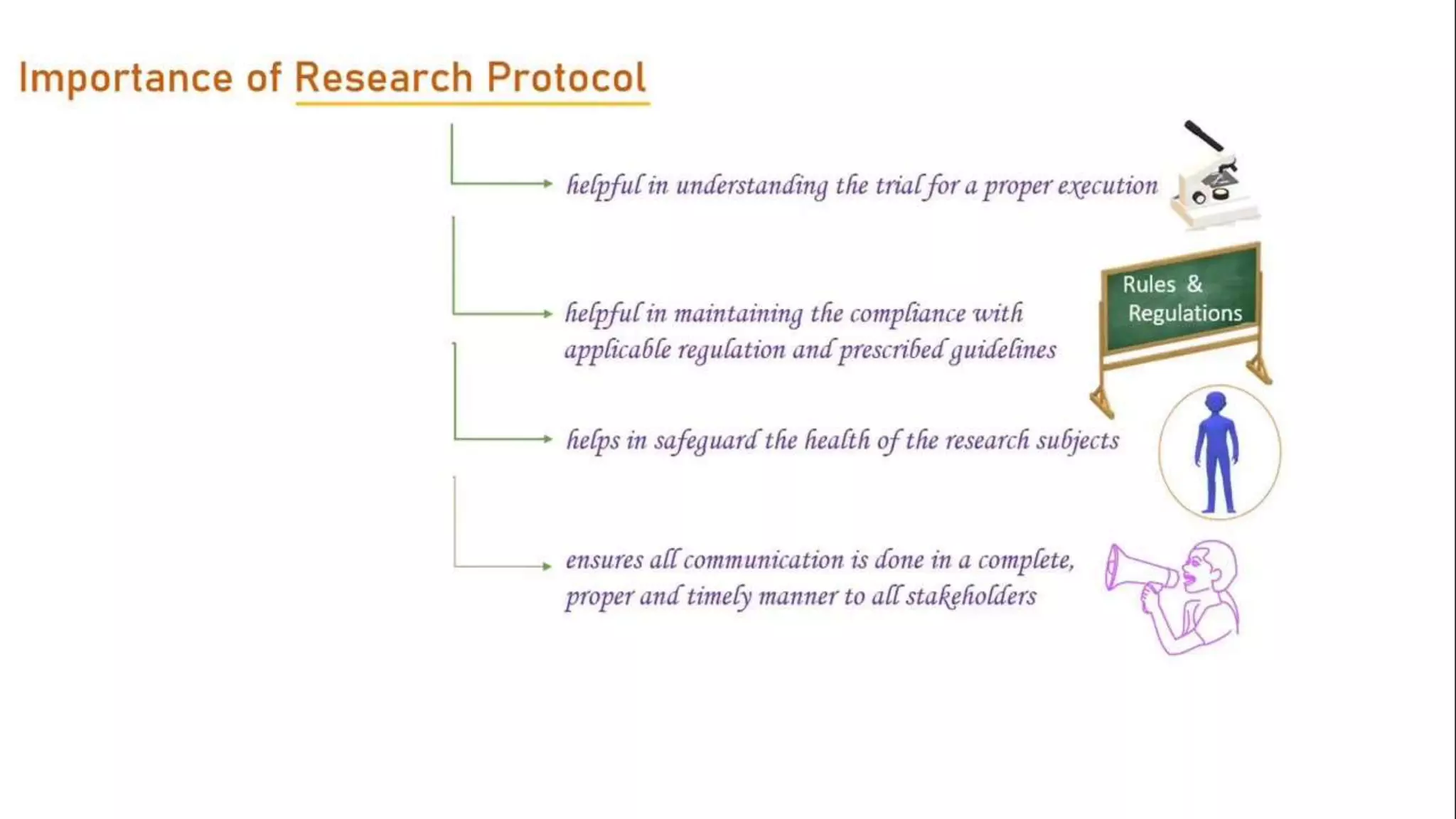
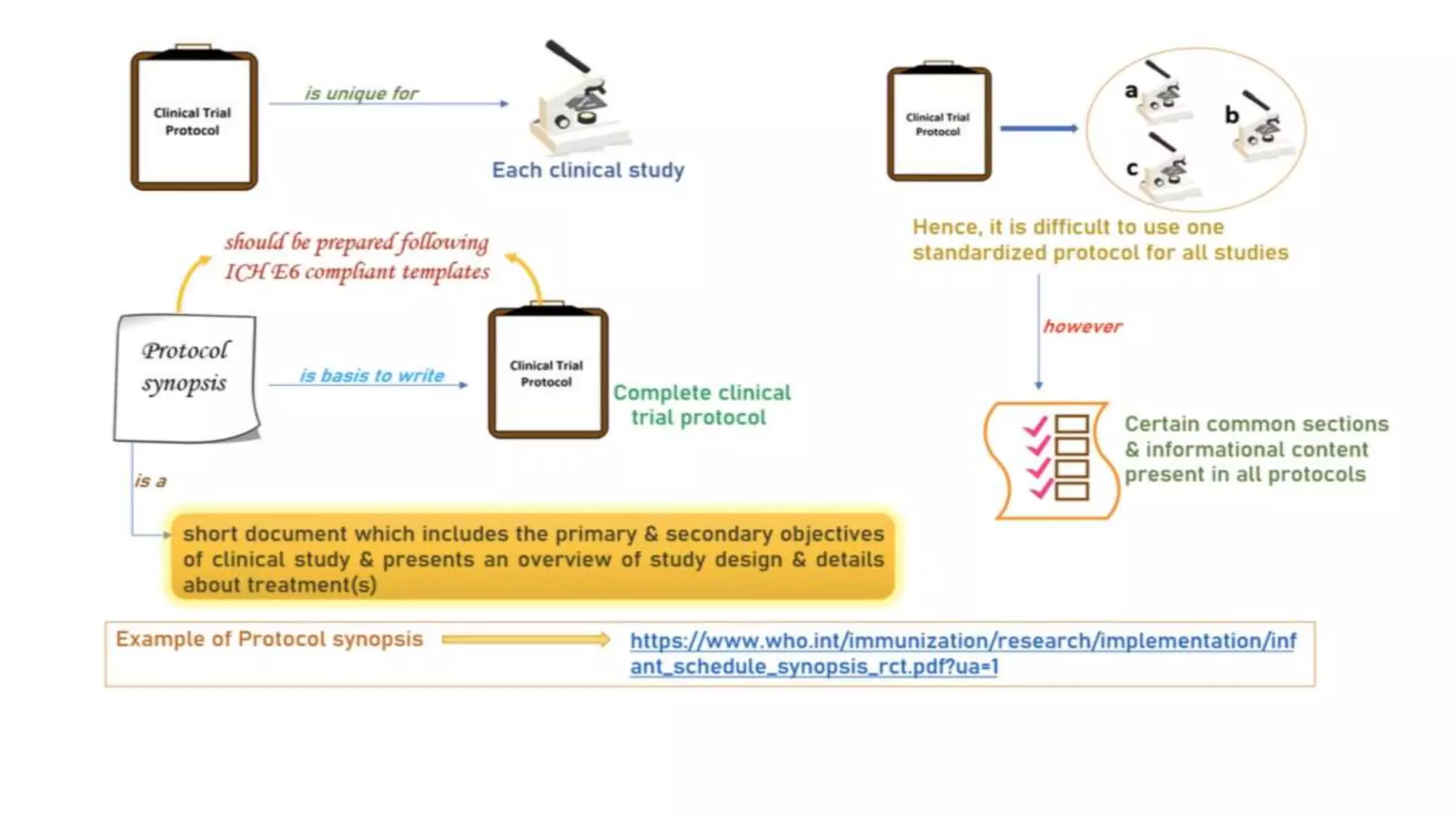
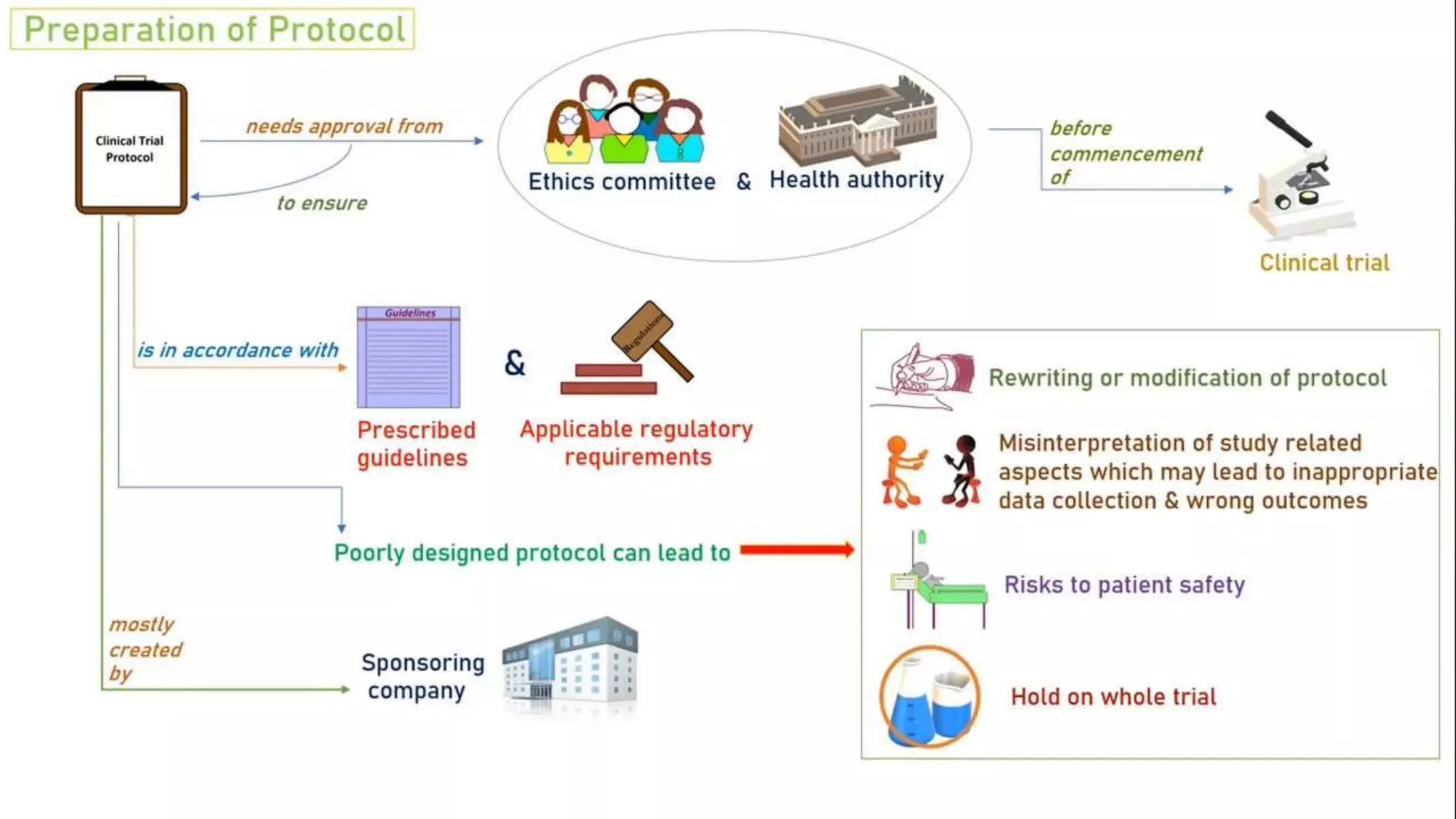


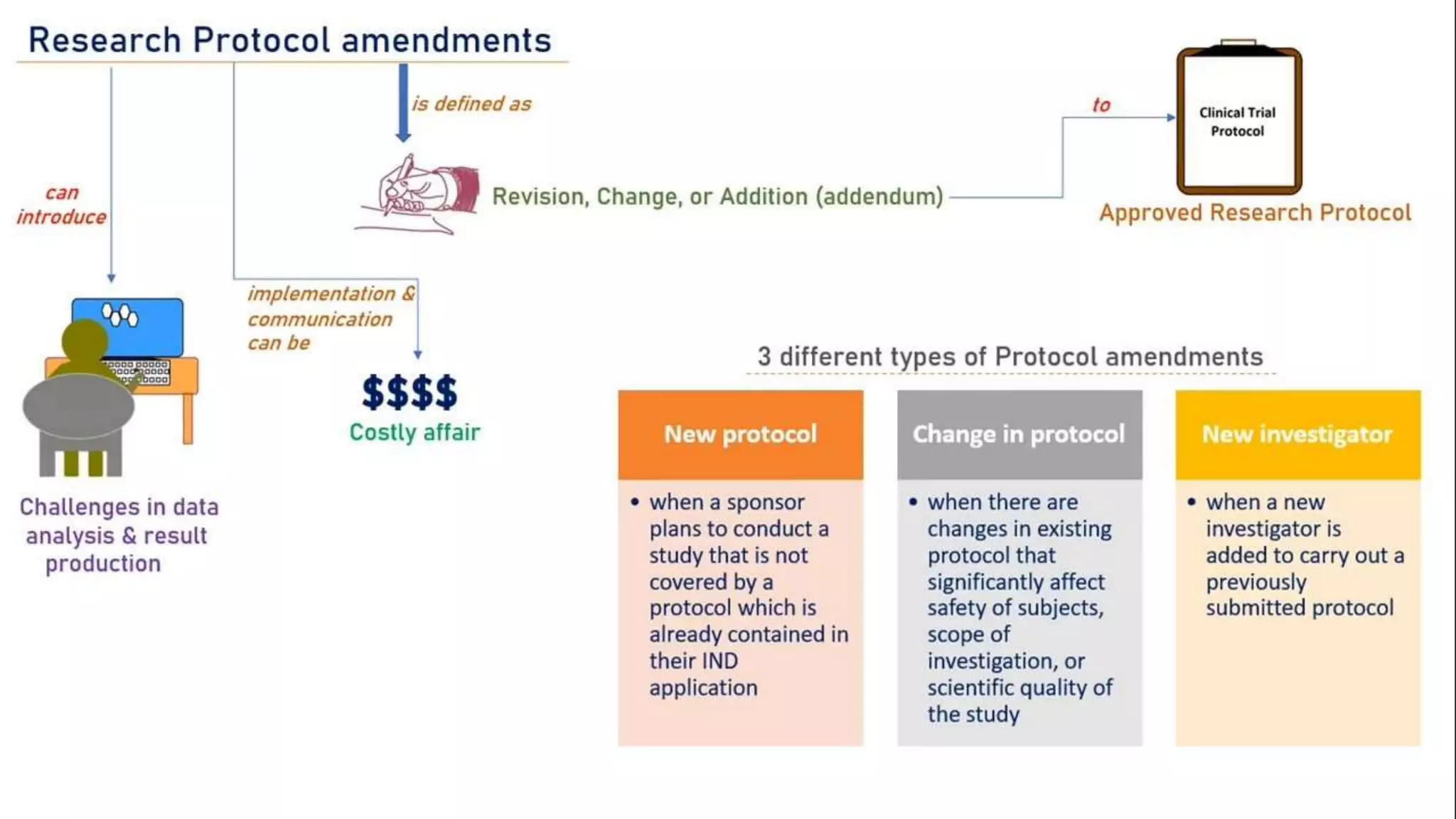


The document discusses ICH-GCP E6 guidelines related to clinical research regulations, focusing on ethical principles, risks vs benefits, and the rights and safety of participants. It includes key components such as the roles of institutional review boards, sponsors, and investigators, alongside essential documents for conducting clinical trials. Additionally, it emphasizes compliance, informed consent, confidentiality, and quality assurance in clinical research.









































































