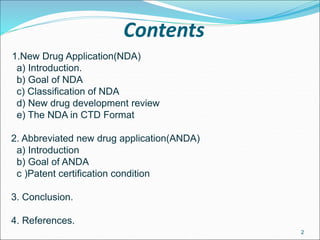
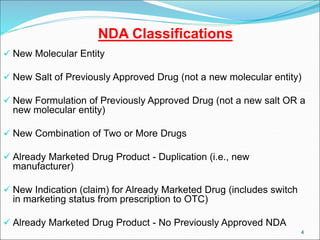
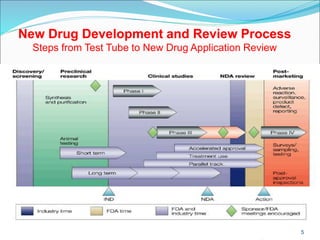
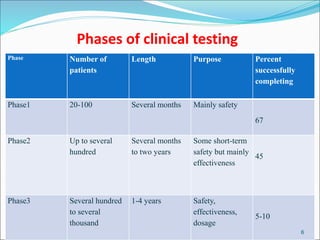
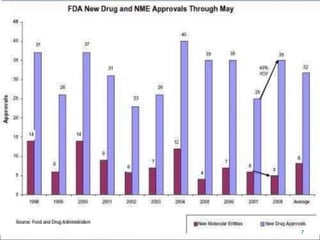
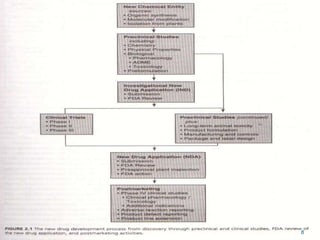
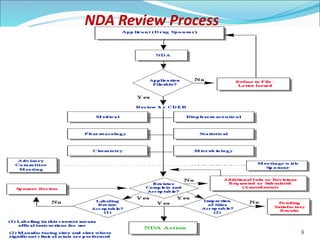
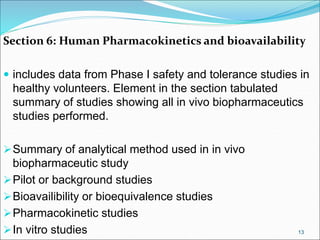
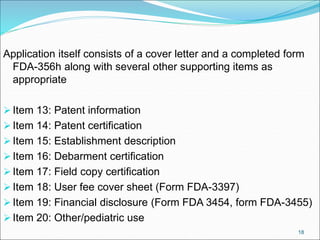
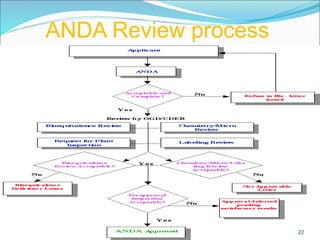
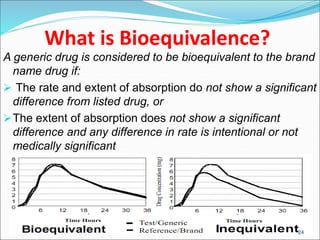
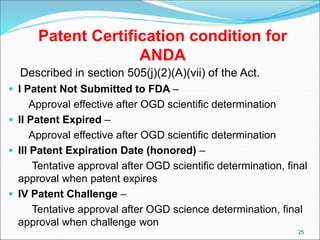
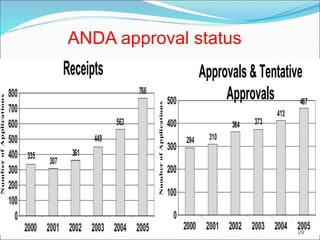
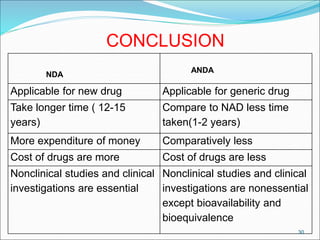
This document provides an overview of New Drug Applications (NDAs) and Abbreviated New Drug Applications (ANDAs). NDAs are required for new drugs and include extensive clinical trial data to prove safety and efficacy. The review process takes 12-15 years and is more expensive. ANDAs are for generic drugs and do not require new clinical trials, only proof of bioequivalence. The review process takes 1-2 years and is less expensive. Both application types provide information on chemistry, manufacturing, labeling, and clinical data in a standardized format.