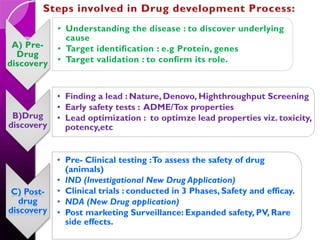
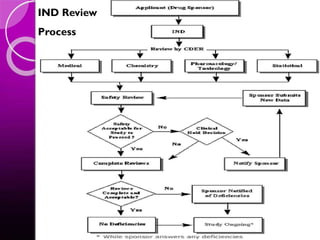
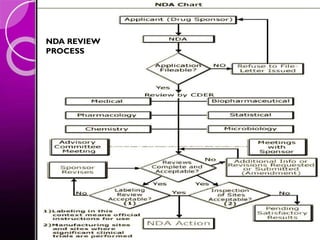
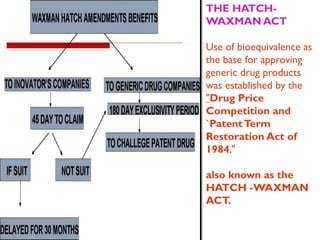
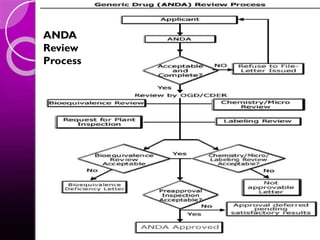
The document discusses the drug development process, outlining stages from pre-drug discovery to post-drug marketing, emphasizing the importance of regulatory submissions such as the Investigational New Drug Application (IND) and New Drug Application (NDA). It details definitions relevant to the industry and explains various FDA regulations and timelines for drug approval, including the Abbreviated New Drug Application (ANDA) for generics. The content underscores the critical role of regulatory bodies in ensuring that drugs are both safe and effective before reaching the market.