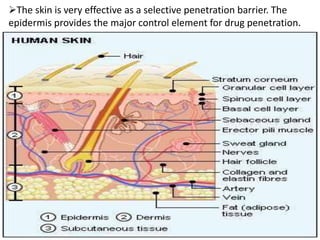
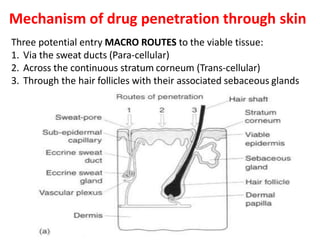
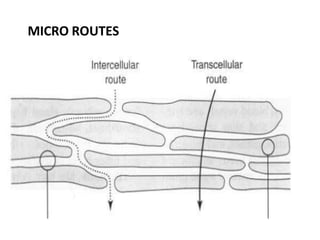
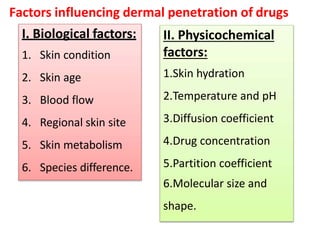
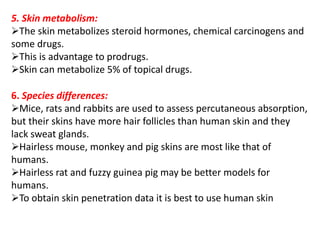
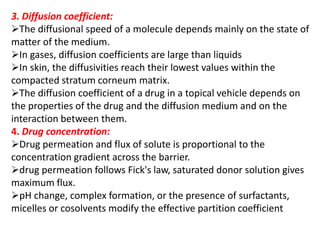
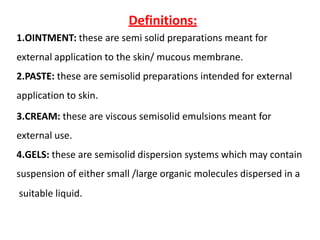
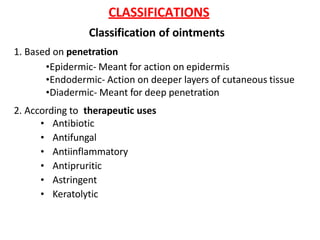
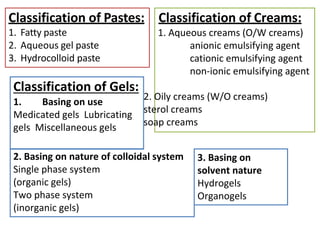
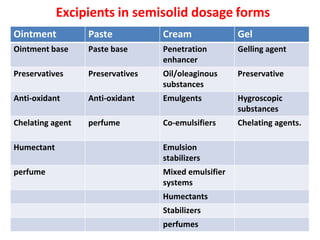
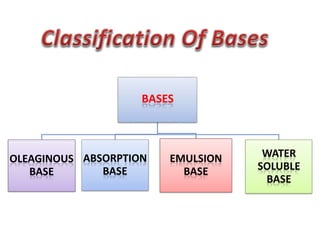
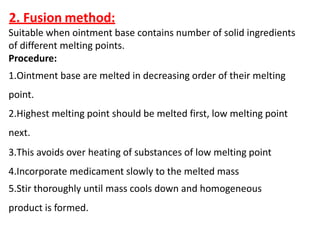
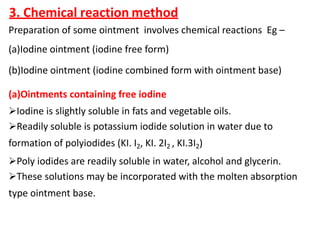
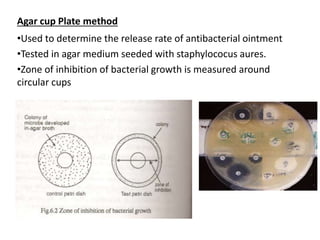
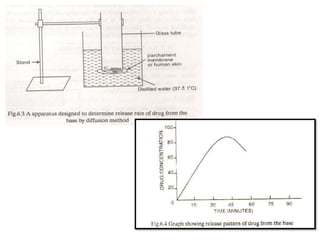
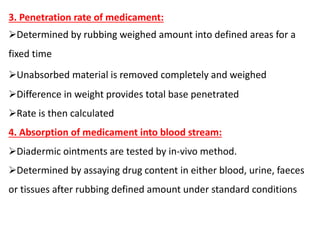
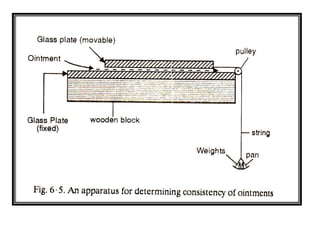
This document discusses semi-solid dosage forms such as ointments, pastes, creams, and gels used for dermal drug delivery. It describes the three potential routes of drug penetration through the skin including via sweat ducts, across the stratum corneum, and through hair follicles. Factors that influence dermal penetration include biological factors like skin condition, age, and blood flow as well as physicochemical factors like skin hydration, temperature, drug concentration, and molecular size. Common bases used for semi-solid formulations are discussed including oleaginous, absorption, emulsion, and water soluble bases. Excipients commonly included in these formulations and their purposes are also outlined.