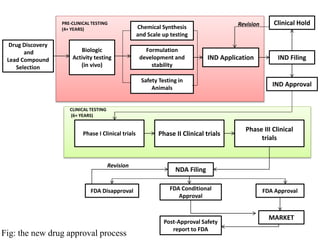
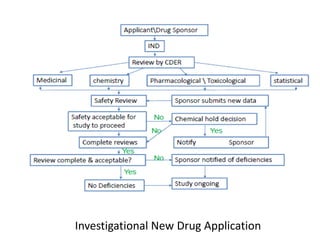
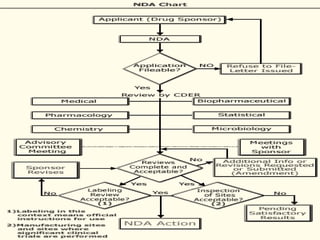
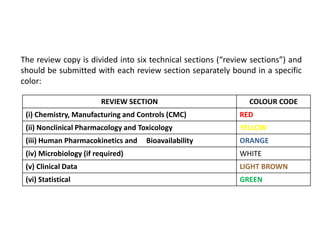
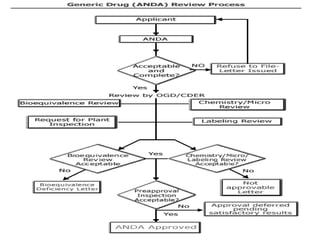
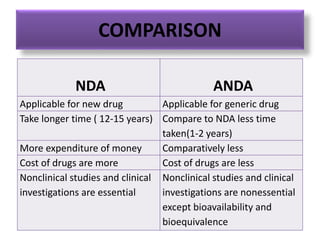
This document provides an overview of regulatory requirements for drugs and pharmaceuticals. It discusses drug regulation by international agreements and regulatory authorities like the FDA. The FDA is organized into centers responsible for drugs, biologics, devices and food. Applications like IND, NDA, ANDA are described which are required for investigational and approved drugs. The new drug approval process is outlined involving pre-clinical and clinical testing taking 10+ years. Key differences between NDA for new drugs and ANDA for generic drugs are summarized.