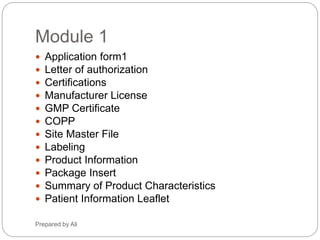
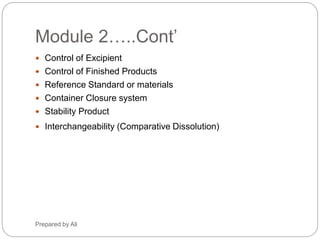
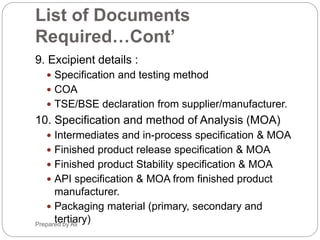
The document provides an overview of the requirements and guidelines for generic drug dossiers using the ASEAN Common Technical Document (ACTD) format. It describes the organization of the ACTD, which contains four parts covering administrative data, quality documents, nonclinical documents, and clinical documents. Part I includes the table of contents, administrative information, and product information. Part II focuses on quality documents for the drug substance and product. Parts III and IV are generally not applicable for generic drugs. The document also lists the types of documents required to complete each part of the ACTD for a generic drug application.