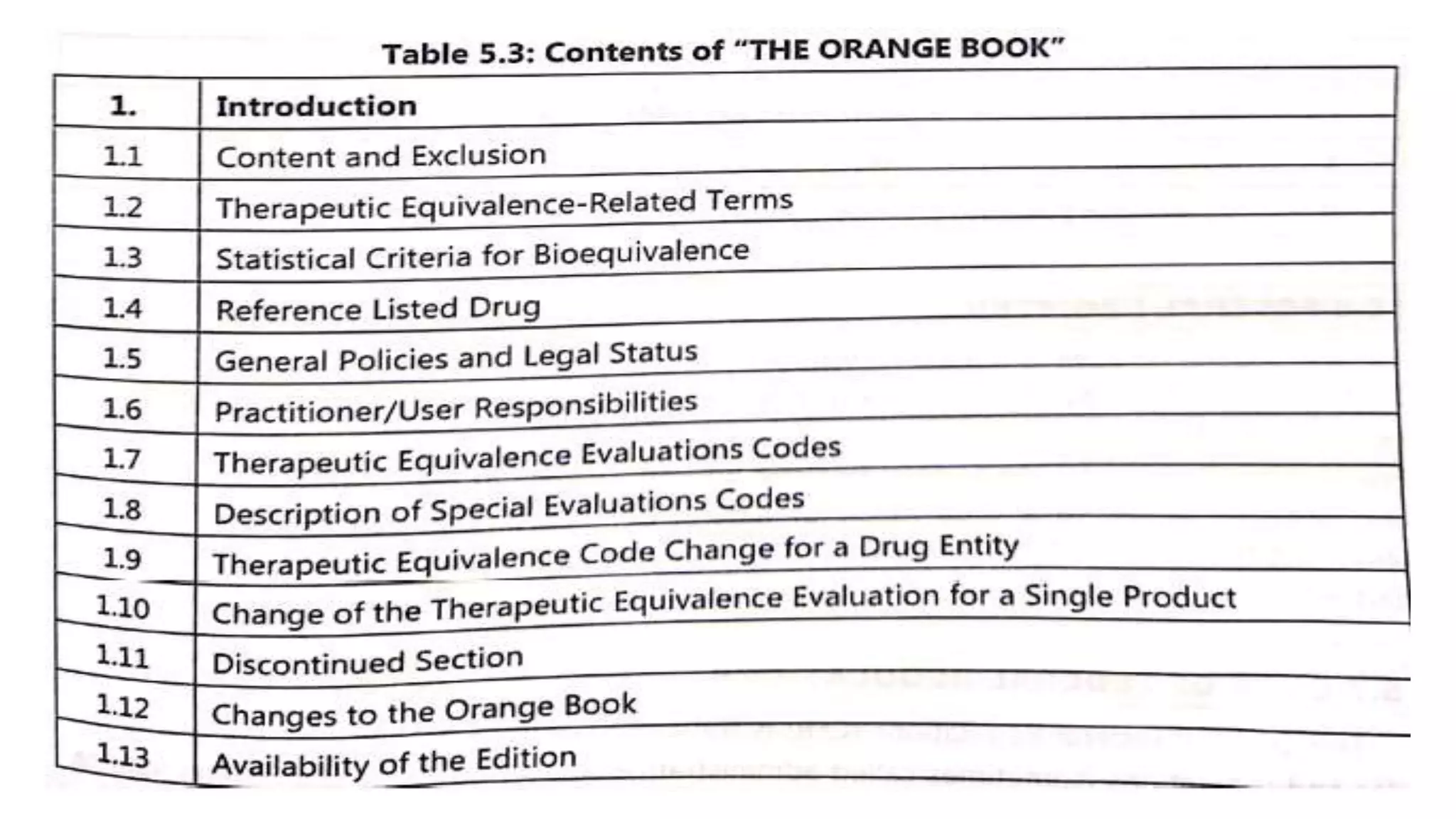
The document outlines key regulatory concepts in pharmaceutical science, including the roles and applications in drug registration, evaluation, and marketing authorization processes. It details various application types like IND, NDA, ANDA, and MAA along with their respective purposes and procedures. Additionally, it discusses significant legislation such as the Hatch-Waxman Act and other compliance guidelines shaping the regulatory landscape for pharmaceutical products.