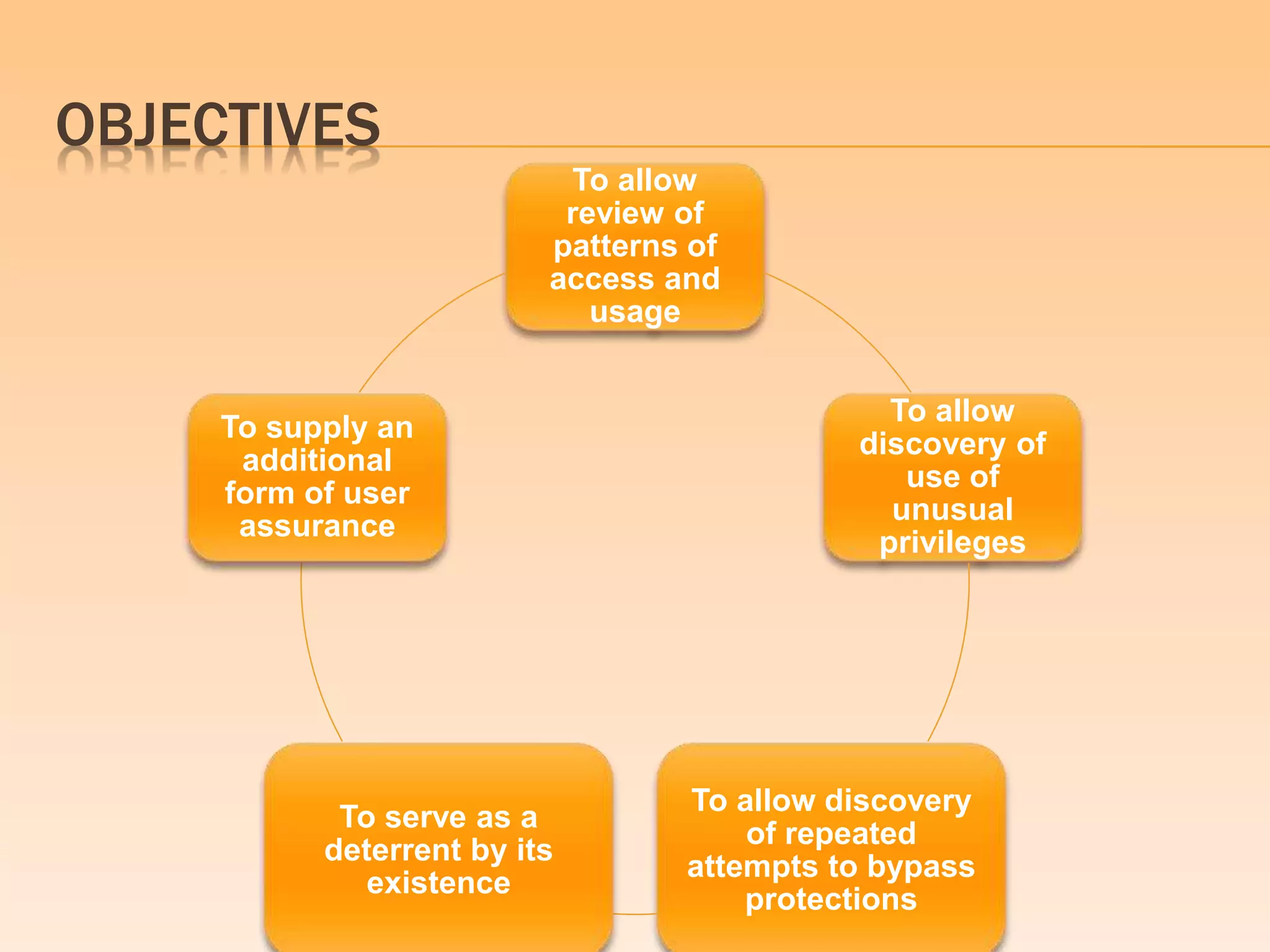
This document provides an introduction and overview of the Orange Book, formally known as "Approved Drug Products with Therapeutic Equivalence Evaluations". The Orange Book is published by the FDA and lists approved drug products and determines whether generic drugs are equivalent to brand name drugs. It identifies drug products approved by the FDA, determines therapeutic equivalence of generic drugs, and provides patent and exclusivity information to facilitate generic drug approval. The document outlines the history, contents, and objectives of the Orange Book in facilitating review of drug use and substitution of equivalent generic drugs.