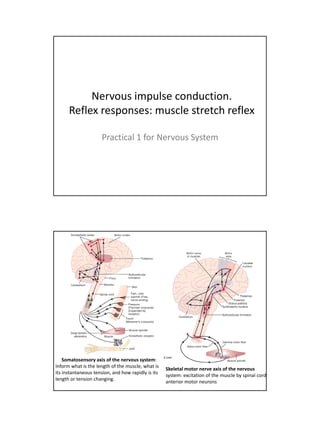
Muscle stretch reflex
- 1. Nervous impulse conduction. Reflex responses: muscle stretch reflex Practical 1 for Nervous System Somatosensory axis of the nervous system: Inform what is the length of the muscle, what is Skeletal motor nerve axis of the nervous its instantaneous tension, and how rapidly is its system: excitation of the muscle by spinal cord length or tension changing. anterior motor neurons
- 2. Sensory signals enter th cord th S i l t the d through th sensory ( h the (posterior) roots. t i ) t After entering the cord, every sensory signal travels to two separate destinations: (1) One branch of the sensory nerve terminates almost immediately in the gray matter of the cord and elicits local segmental cord reflexes and other local effects. (2) Another branch transmits signals to higher levels of the nervous system - to higher levels in the cord itself, to the brain stem, or even to the cerebral cortex 1 - sensory transducer in the periphery for example a Pacinian corpuscle or other periphery, example, tactile sensor in the skin. 2 - the pseudounipolar sensory neuron in the circuit. Its soma is physically located in a dorsal root ganglion 3 - an interconnector neuron, whose soma is found in the CNS. 4 - motor neuron whose soma is in the ventral horn of the gray H of the spinal cord. 5 - the effector organ, which in the case of this type of arc, will always be skeletal muscle.
- 3. Somatic arc reflex: how the system works? 1. Something impinges on the transducer, which causes the afferent fiber of the pseudounipolar sensory neuron to fire. 2. That signal is transmitted via the sensory neuron efferent fiber into the CNS, specifically into a synapse with an interconnector neuron in the dorsal horn of the gray H. 3. That neuron then sends a signal to a synapse with the motor neuron in the ventral horn. 4. The axon of the motor neuron - which may actually be several meters in length - l i l th leaves th CNS and t the d terminates at a motor end plate on i t t t d l t some myofiber. When it fires it initiates contraction. ! This loop is completely independent; it's not necessary to have CNS involvement beyond the "relay" at the interconnector neuron. Ex: What happens when you inadvertently put your hand on a hot stove burner…. Axons conduct action potentials away from the cell body of the nerve cell Dendrites receive action potentials
- 4. Nerve Conduction Nerve Conduction Velocity Along the Ulnar Nerve of a Human Subject The nerve impulse is a wave of depolarization immediately followed by a wave of repolarization, collectively called an action potential, occurring on the plasma membrane of a nerve fiber. Changes in ion conductances across the nerve fiber membrane are responsible for the initiation and propagation of the action potential. Experimentally, these changes can be the result of electrical current applied through electrodes. Once initiated, an action potential is usually propagated without decrement in amplitude or velocity along the plasma membrane of a nerve fiber. The velocity or speed of the propagated (conducted) nerve impulse is directly related t the diameter of th nerve fib and th presence of a myelin l t d to th di t f the fiber d the f li sheath. • The fastest nerve fibers have large diameters and are myelinated (motor nerve fibers for skeletal mm). • The slowest nerve fibers have small diameters and are unmyelinated (sensory nerve fibers).
- 5. In the peripheral nervous system, nerve fibers of various diameters and functions (motor and sensory) are bundled together by connective tissue to form nerves. A compound action potential is the sum of all the action potentials occurring in the individual neurons of the whole nerve. The velocity of the compound action potential signal can be a measure and can indicate the state of health of the nerve. Diseases that damage the th myelin, destroy neurons, or constrict the whole nerve will d li d t t i t th h l ill decrease the nerve's conduction velocity. However, the nerve conduction velocity may remain normal until late in a disease process as long as a few normal neurons survive, In addition, the nerve conduction velocity reflects conduction of the fastest nerve fibers, usually motor neurons. The nerve conduction velocity is determined by recording the motor (EMG) response of a muscle to the stimulation of its motor nerve at two or more points along the nerve course. The time between stimulation and response is measured and compared to the distance between the point of stimulation and point of response. Precise parameters of measurement have been developed for the ulnar nerve and the motor response is measured over the abductor digiti minimi. The ulnar nerve comes from the medial cord of the brachial plexus, and runs inferior on the medial/posterior aspect of /p p the humerus down the arm, going behind the medial epicondyle, through the cubital tunnel, at the elbow (where it is exposed for a few centimeters, just above the joint).
- 6. Objectives 1. 1 To record a charge from the stimulus electrodes to recording electrodes. 2. To observe the Threshold, Maximal and Supra-Maximal response levels. 3. To determine nerve conduction velocity along the ulnar nerve. Equipment • PC running Windows or Macintosh computer • BIOPAC Software: Biopac Student Lab PRO • BIOPAC Data Acquisition Unit (MP30) • BIOPAC Stimulator (BSLSTM) • BIOPAC disposable electrodes (EL503) • BIOPAC electrode lead set (SS2L) • BIOPAC human stimulator probe (HSTM01) • Abrasive pads (ELPAD) • Electrode gel (GEL1) • Ruler (cm) • Adhesive Tape (TAPE1) • Pen for marking skin
- 7. Setup Hardware 1. Plug the BSLSTM Trigger cable into the Analog Out port on the back panel of the MP 30 Unit. 2. Plug the BSLSTM Reference Output cable into CH1 on the front of the MP30 unit. 3. Plug the SS2L electrode lead into CH2 on the front of the MP30 Unit. 4. Turn the MP30 Data Acquisition Unit on. 5. Turn the BSLSTM Stimulating Unit on. 6. Set the BSLSTM Range to "0-100V" and Level to "0" by turning the Level knob counterclockwise until it stops 7. Plug the HSTM01 human stimulator probe into the Stimulus Output port on the front of the BSLSTM Unit. Setup Software 1. Turn the computer on. 2. Launch the BSL PRO software on the host computer. The program should create a new "Untitled1" window. You may close this window, you will not need it. 3. Open the Nerve Conduction Velocity template by choosing File menu > Open > Then change the FILE TYPE to: Graph Template (*GTL) and locate the File Name: h03.gtl on the desktop of the computer. This should open a data acquisition window and the Stimulator control window, as shown below. 4. Click on the Display menu, then select Show and select Markers. This will add a white text box directly above the top data collection panel. This is where you can type notes about each stimulus that you administer.
- 8. Subject - Electrode Connections 5. Remove any jewelry or watches on your wrist. 6. Clean skin with an alcohol prep and allow to air dry before proceeding. 7. Apply a very small drop of electrode gel (GEL1) in the center of each electrode. 8. Place three EL503 disposable recording electrodes on the subject's hand as follows and attach the appropriate colored electrode leads to each of the electrode sites; use the cloth tape provided to secure each electrode tightly to the skin making sure to not cover up the metal electrode pin connections 9. Clip the SS2L electrode cable to the subject’s shirt or clothes to relieve cable strain and avoid pulling on the electrodes.
- 9. Experimental Method You will record the stimulation of the ulnar nerve at two points along the forearm. Note the stimulation points on the figure below. You may have to move around and adjust the electrode placement to find the optimal placement. NOTE: The white anode electrode is always further from the hand and the black cathode electrode is always closest to the hand. (we will not use the S3 stimulation position) Experimental Method 1. The subject should hold the HSTM01 stimulating probe at position S1 and depress and hold the red button down for stimulation to occur. 2. Increase the level setting on the BSLSTM unit to 5V. 3. When ready, the experimenter should press the Start button in the PRO software indo soft are window (this will begin stim lation and 200msec data acq isition) E er ill stimulation acquisition). Every time that you administer a stimulus by pressing the start button, a marker with a timestamp will be automatically inserted for you. You may add notes to this timestamp. 4. Increase the level setting on the BSLSTM unit by 5V increments until a response is detected. • A response may be indicated by involuntary twitching of the fingers. • A response typically occurs between stimulus levels of 25 to 40 volts. p yp y • Move the probe around a little if no response occurs by 45 volts. • If the subject experiences pain with the finger twitch, and a response has not been detected, move the stimulating probe to a new position. A response should be detected well before the subject experiences any pain. • The recorded response on Channel 40 (blue line, bottom panel) may have one or two upward peaks followed by a downward deflection.
- 10. 5. Once you have detected a response, adjust the electrode placement to make sure that you had the optimal placement. Then mark with a small ink dot the location of the black cathode electrode. 6. Administer 3 stimuli at the S1 location and label each stimulus in the marker box as S1-1 S1-2 S1-3 S1 1, S1 2, S1 3. 7. Now reposition the stimulating electrode at the S2 position after applying more gel to this location. Decrease the voltage to 5V less than the value for S1 and repeat the testing process. Be sure to mark the placement of the black cathode electrode once you have achieved a response. Record 3 stimuli (label these in the marker text box) for the S2 location. 8. Finally, measure the distance in centimeters from the S1 to S2 cathode placements Record this distance. 9. You have completed the data collection process and may now disconnect the electrodes, turn off the stimulator and BIOPAC MP30 devices, and proceed with cleaning up the equipment and subject. Data Analysis At the conclusion of your recording session you may want to make your data collection measurements or you can return at a later date to collect your data. The data collection measurements should already be appropriately selected for you in the BIOPAC window. 1. Scroll through the data until you locate your first stimulus for S1. By clicking on the inverted triangle markers located above the data window you can view the marker text associated with each marker position. 2. Once you find your Threshold stimuli, you will need to change from the “arrow” cursor to the I-beam curser by selecting the I-beam button in the bottom right corner of the display. 3. Use the curser to select the data from the start of the stimulus (red panel) to the peak of the response (blue panel) as shown in the example figure above. 4. These data variables are already set-up for you in the boxes above the data signal: Ch1 p-p (peak-to-peak) value – this is the voltage applied Ch40 p-p value – this is the magnitude of the stimulation in the ulnar nerve Ch40 delta T value – this is the duration of time between the stimulus and the nerve signal detected by the recording electrode
- 11. 5. Record the Ch40 delta T value from the onset of the voltage (red panel) to the peak of the ulnar nerve stimulation (blue panel) for each of the S1 stimuli. You may need to zoom in using the magnifying glass tool in order to see the data in enough detail to accurately measure the time. 6. Enter the delta T values for S1, S2 and your measured distance between the cathode electrodes for S1 & S2 into a data EXCEL sheet found on the lab web site. The excel sheet will calculate the speed of conduction using the following formula: (Distance between S1 and S2) / (delta T for S2 - delta T for S1) 7. You 7 Y need t i d to input th speed of conduction values ( h t the d f d ti l (shown i red t t i th E in d text in the Excel l sheet) into the group data sheet found on the desktop of the computer.
- 12. Muscle stretch reflex Monosynaptic pathway that allows a reflex signal to return with the shortest possible time delay back to the muscle after excitation of the spindle Neuronal circuit of the muscle stretch reflex - the simplest manifestation of muscle spindle function. Whenever a muscle is stretched suddenly, excitation of the spindles causes reflex contraction of the large skeletal muscle fibers of the stretched muscle and also of closely allied synergistic muscles.
- 13. The dynamic stretch reflex is elicited by the potent dynamic signal transmitted from the primary sensory endings of the muscle spindles, caused by rapid stretch or unstretch. That is, when a muscle is suddenly stretched or unstretched, a strong signal is transmitted to the spinal cord; thi causes an i t t i l d this instantaneous strong reflex contraction t fl t ti (or decrease in contraction) of the same muscle from which the signal originated. Thus, the reflex functions to oppose sudden changes in muscle length. The dynamic stretch reflex is over within a fraction of a second after the muscle has been stretched (or unstretched) to its new length, but then a ( ) g , weaker static stretch reflex continues for a prolonged period thereafter. This reflex is elicited by the continuous static receptor signals transmitted by both primary and secondary endings. This static stretch reflex causes the degree of muscle contraction to remain reasonably constant, except when the person's nervous system specifically wills otherwise. Muscle Sensory Receptors: Muscle Spindles and Golgi Tendon Organs Muscle function requires not only excitation of the muscle by spinal cord anterior motor neurons but also continuous feedback of sensory information from each muscle to the spinal cord, indicating the functional status of each muscle at each instant (length of the muscle, instantaneous tension, how rapidly is its length or ( g p y g tension changing). Muscles and their tendons are supplied abundantly with two special types of sensory receptors: (1) muscle spindles, which are distributed throughout the muscle and send information to the nervous system about muscle length or rate of change of length, (2) Golgi tendon organs, which are located in the muscle tendons and transmit information about tendon tension or rate of change of tension. The signals from these two receptors are almost entirely for the purpose of intrinsic muscle control. They operate almost completely at a subconscious level and transmit information not only to the spinal cord but also to the cerebellum and even to the cerebral cortex, helping to the control of muscle contraction.
- 14. Muscle spindles are distributed throughout the belly of the muscle, send information to the NS about muscle length or rate of change of length; both motor and sensory innervation. Each spindle is 3 ‐ 10 mm long. It is built around 3 ‐ 12 very small intrafusal muscle fibers that are pointed at their ends and attached to the glycocalyx of the surrounding large extrafusal skeletal muscle fibers fibers. Each intrafusal muscle fiber is a very small skeletal muscle fiber, with: ‐ a central region with few or no actin and myosin filaments (does not contract when the ends do), that functions as a sensory receptor. ‐the end portions that do contract and are excited by small gamma motor /efferent fibers that originate from small type A gamma motor neurons in the anterior horns of the spinal cord. Extrafusal skeletal muscle is innervated by large alpha efferent fibers (type A alpha nerve fibers), that branch many times after they enter the muscle and innervate from three to several hundred skeletal muscle fibers of the same motor unit. Nerve connections from the nuclear bag & nuclear chain muscle spindle fibers. Muscle spindle receptor can be excited in two ways: - Lengthening the whole muscle stretches the midportion of the spindle and, therefore, excites the receptor. - Even if the length of the entire muscle does not change, contraction of the end portions of the spindle's intrafusal fibers stretches the midportion of the spindle and therefore excites the receptor.
- 15. Golgi tendon organ helps control muscle tension: encapsulated sensory receptor through which muscle tendon fibers pass. About 10 ‐ 15 muscle fibers are usually connected to each Golgi tendon organ, and the organ is stimulated when this small bundle of muscle fibers is "tensed" by tensed contracting or stretching the muscle. Thus, the major difference in excitation of the Golgi tendon organ versus the muscle spindle is that the spindle detects muscle length and changes in muscle length, whereas the tendon organ detects muscle tension as reflected by the tension in itself. Motor units
- 16. REFLEX RESPONSE "Damping" Function of the Dynamic and Static Stretch Reflexes Stretch reflex is able to prevent oscillation or jerkiness of body movements movements. This is a damping, or smoothing function. Damping mechanism in smoothing muscle contraction. Signals from the spinal cord are often transmitted to a muscle in an unsmooth form, increasing in intensity for a few milliseconds, then decreasing in intensity, then changing to another intensity level, and so forth. When the muscle spindle apparatus is not functioning satisfactorily, the muscle contraction is jerky during the course of such a signal.
- 17. The damping mechanism's ability to smooth muscle contractions, even though the primary input signals to the muscle motor system may themselves be jerky. This effect can also be called a signal averaging function of the muscle spindle reflex. Muscle contraction caused by a spinal cord signal under two conditions: curve A, in a normal muscle (the muscle spindle reflex of the excited muscle is intact; the contraction is relatively smooth, even though the motor nerve to the muscle is excited at a slow frequency of only 8 signals per second. ), and curve B, in a muscle whose muscle spindles were denervated by section of the posterior roots of the cord 82 days previously; note the unsmooth muscle contraction. Clinical Applications of the Stretch Reflex Almost every time a clinician performs a physical examination on a patient, he or she elicits multiple stretch reflexes. The purpose is to determine how much background excitation, or "tone," the brain is sending to the spinal cord. This reflex is elicited as follows. follows Knee Jerk and Other Muscle Jerks. Clinically, a method used to determine the sensitivity of the stretch reflexes is to elicit the knee jerk and other muscle jerks. The knee jerk can be elicited by simply striking the patellar tendon with a reflex hammer; this instantaneously stretches the quadriceps muscle and excites a dynamic stretch reflex that causes the lower leg to "jerk" forward. myogram from the quadriceps muscle recorded during a knee jerk. Similar reflexes can be obtained from almost any muscle of the body either by striking the tendon of the muscle or by striking the belly of the muscle itself. In other words, sudden stretch of muscle spindles is all that is required to elicit a dynamic stretch reflex.
- 18. Myograms recorded from the quadriceps muscle during elicitation of the knee jerk (above) and from the gastrocnemius muscle during ankle clonus (below). The muscle jerks are used by neurologists to assess the degree of facilitation of spinal cord j y g g p centers. When large numbers of facilitatory impulses are being transmitted from the upper regions of the central nervous system into the cord, the muscle jerks are greatly exaggerated. Conversely, if the facilitatory impulses are depressed or abrogated, the muscle jerks are considerably weakened or absent. These reflexes are used most frequently in determining the presence or absence of muscle spasticity caused by lesions in the motor areas of the brain or diseases that excite the bulboreticular facilitatory area of the brain stem. Ordinarily, large lesions in the motor areas of the cerebral cortex but not in the lower motor control areas (especially lesions caused by strokes or brain tumors) cause greatly exaggerated muscle jerks in the muscles h d f h b d