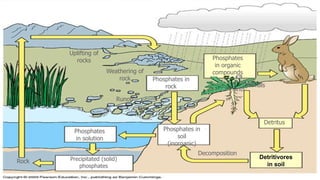
The document discusses the phosphorus cycle and transformations of phosphorus within soil. It notes that phosphorus is essential for life but cannot exist as a gas, and its cycle is the slowest. Within soil, microbes play an important role in mineralizing organic phosphorus compounds into a form plants can absorb through enzymes. They also solubilize insoluble phosphates and precipitate phosphorus compounds. The transformations involve acids, gases, chelators and are impacted by pH, calcium, magnesium, iron and aluminum levels.