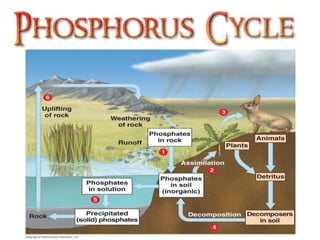
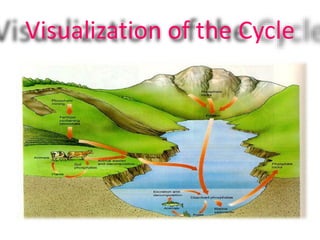
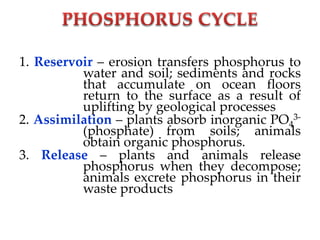
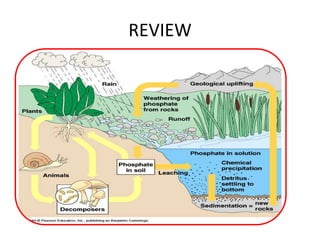
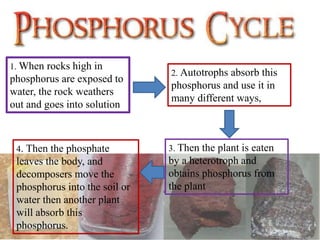
Phosphorus was discovered in 1669 by Hennig Brand in Germany. It exists naturally as phosphate minerals in the earth's crust and is essential for all life as a component of DNA, RNA, ATP, and bones. Phosphorus cycles through the lithosphere, hydrosphere, and biosphere as it weathers from rocks into soil and water, is absorbed by plants and animals, and returns to the environment through decomposition. Human activities like phosphate fertilizer production and forest clearing have disrupted the natural phosphorus cycle.