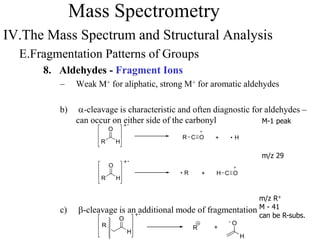
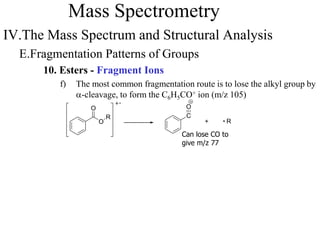
Mass spectrometry involves ionizing molecules and separating the resulting ions based on their mass-to-charge ratio. The technique can determine molecular mass and formula. It works by bombarding molecules with electrons to produce radical cations, accelerating the ions in an electric field to separate them based on m/z, and detecting the ions. Isotopic abundances provide additional structural information, such as the number of carbon or halogen atoms present.