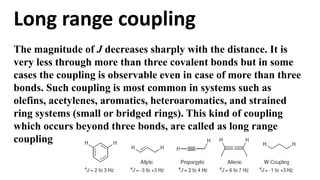
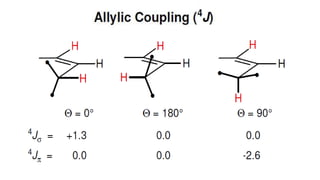
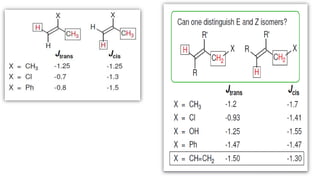
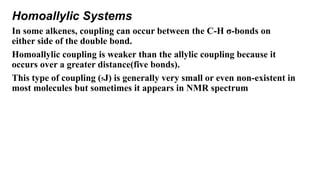
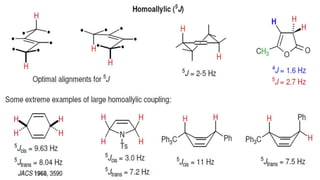
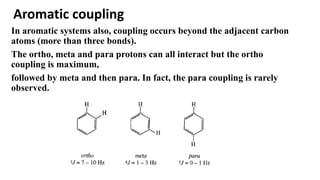
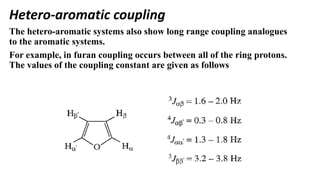
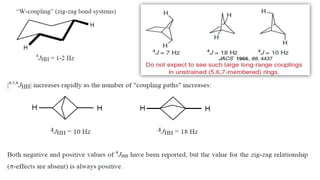
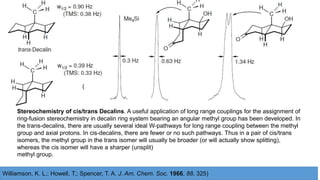
Long range NMR spin-spin coupling can occur beyond three bonds in systems with pi electrons like olefins, acetylenes, aromatics, and heteroaromatics. Specific types of long range coupling discussed include allylic coupling between protons on carbons adjacent to a double bond, homoallylic coupling between protons on either side of a double bond, and aromatic coupling between ortho, meta, and para protons. Rigid systems can also exhibit long range coupling through geometries that allow for orbital overlap like a "W" configuration.