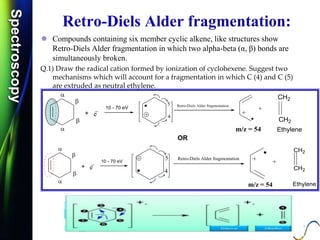
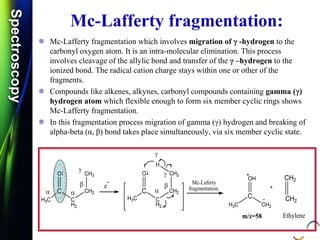
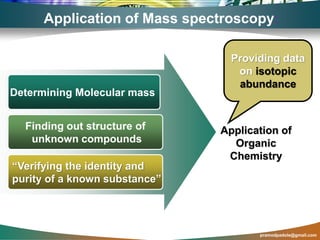
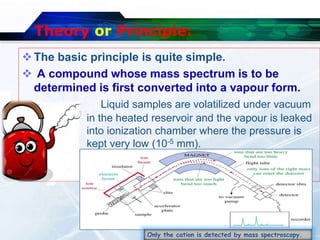
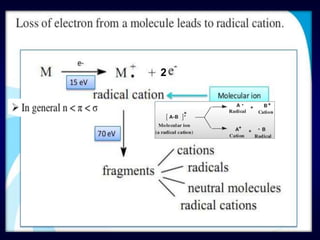
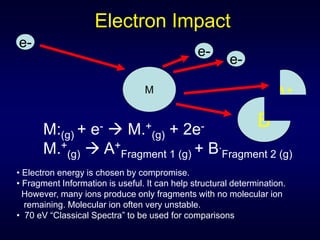
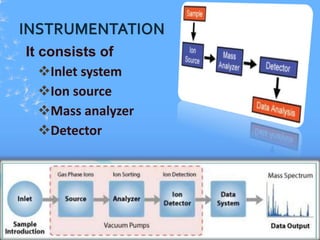
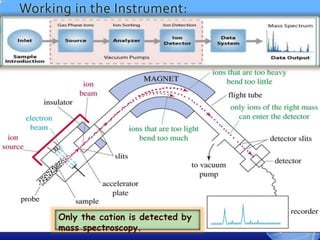
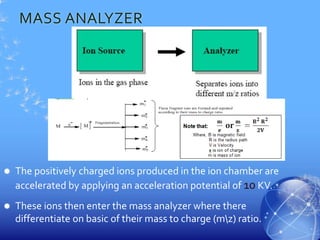
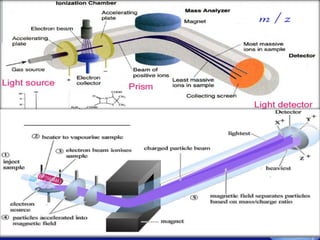
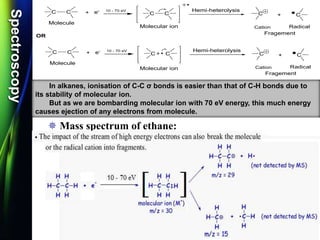
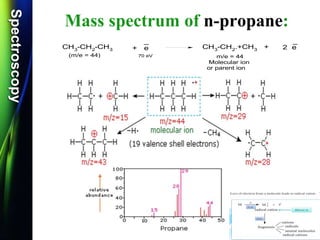
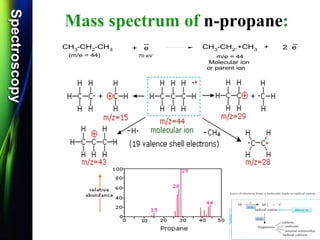
Mass spectroscopy is a technique that determines the molecular mass of compounds by ionizing molecules and measuring their mass-to-charge ratios. It works by first volatilizing and ionizing molecules via electron bombardment, which produces molecular ions. The molecular ions are then accelerated and separated based on their mass-to-charge ratios using electric and magnetic fields. Finally, the ions are detected, and a mass spectrum is produced by plotting the relative abundances of each ion versus the mass-to-charge ratio. Key terms include molecular ion peak, daughter ion peaks, base peak, and metastable ions. Mass spectroscopy is widely used in science to determine molecular structures and isotopic abundances.









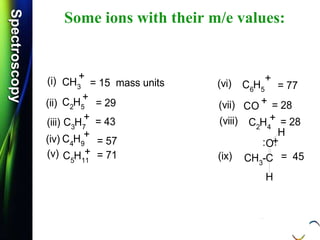




![SpectroscopySpectroscopy
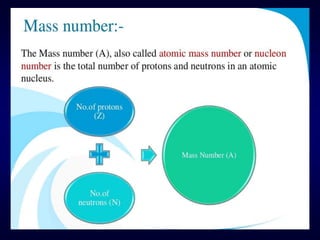
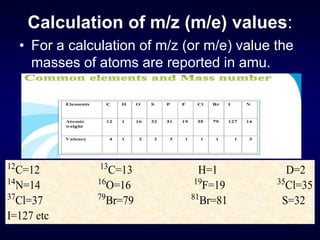
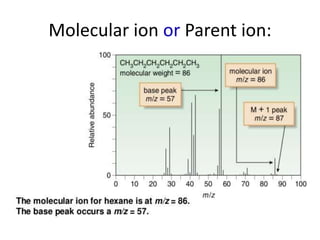
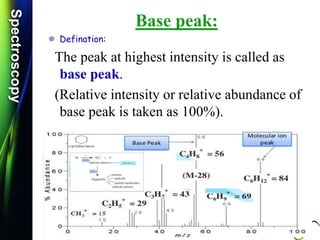
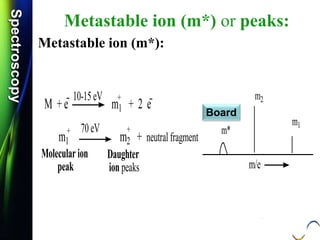
Molecular ion or parent ion:
Defination:
The peak at highest m/e (or m/z) value is
called as molecular ion or parent ion or
radical cation peak.
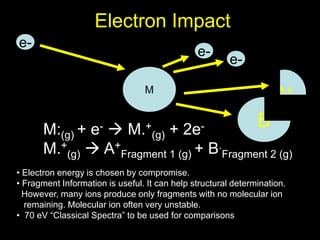
M: + e-
[ M ] + 2 e-
Neutral
Molecule
Molecular Ion
or Parent ion
10 -70 eV +.](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-40-320.jpg)



![SpectroscopySpectroscopy
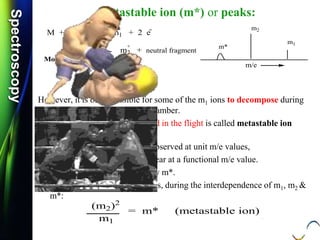
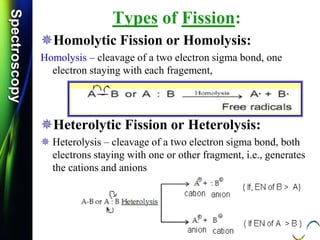
Types of Fission:
Hemi-Heterolytic Fission:
Definition: It is a cleavage of an ionized sigma bond; to
form cation and free radical.
There is only one electron in the bonding sigma orbital,
hence the use of a single headed arrow.
Note that: All molecular ions [cation-radicals] are written
inside square brackets.
Hemi-Heterolysis: This special type of breaking of
ionized sigma covalent bond and
is possible in saturated hydrocarbons.
C C C C+
C C e-
Molecule
Molecular ion
Fragement
Cation Radical
Hemi-heterolysis+ 10 - 70 eV
C C C C+
C C e-
Molecule
Molecular ion
Fragement
Cation Radical
Hemi-heterolysis+ 10 - 70 eV
OR
A B
Hemi-Heterolysis
A B
A B
Hemi-Heterolysis
A B. + .
+](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-53-320.jpg)
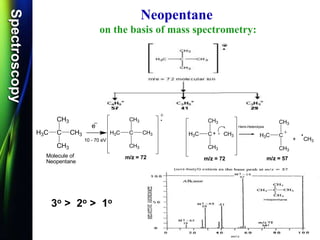
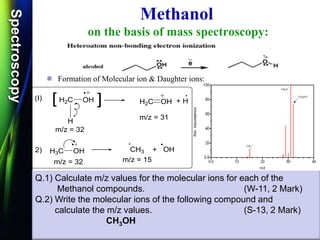
![SpectroscopySpectroscopy
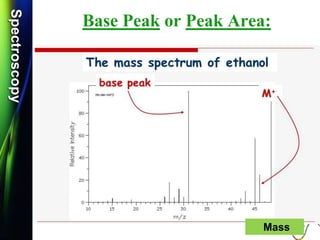
Mass spectrum of Ethanol:
Q.1) Discuss /Explain the mass spectrum of ethanol.
(W-09 & W-14, 2-4 Mark)
Q.2) Calculate m/z values for the molecular ions for each of
the Ethanol compounds. (S-13 & S-14, 2 Mark)
Q.3) Calculate m/z values for the molecular ions for each of
the Methanol compounds. (W-15, 2 Mark)
CH3-CH2-OH
Q.4) Calculate m/z values for the following molecular ion:
(W-16, S-17 & S-19, 2 Mark)
Q.5) Give the structure of a compound C2H6O, whose mass spectrum
shows m/z values of 15, 29, 31 and 46.
(S-18, 4 Mark)
[CH3CH2OH]](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-59-320.jpg)
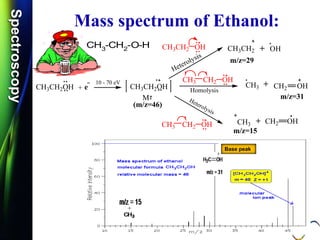
![SpectroscopySpectroscopy
Mass spectrum of Acetone:
Q.1) Discuss /Explain the mass spectrum of acetone.
Q.2) Calculate m/e values for the molecular ions for each of
the Acetone compounds. (S-11, S-12 & S-14, 2 Mark)
Q.3) Write the molecular ions of the following compound and
calculate the m/e values. (S-12, S-14 & W-14, 2 Mark)
CH3COCH3
Q.4) Calculate m/z values for the molecular ions of the
Acetone compound. (W-11, 2 Mark)
Q.5) Calculate m/z values for the following molecular ion:
[CH3COCH3]+. (S-17 & W-17, 2 mark)
Q.6) Discuss the fragmentation of Acetone. (S-18, 4 Mark)
CH3C O
+.
..](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-61-320.jpg)
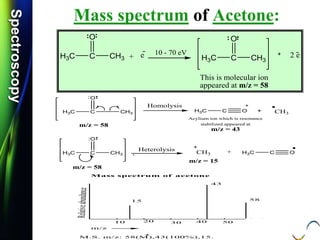
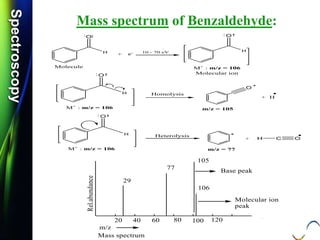
![SpectroscopySpectroscopy
Mass spectrum of Benzaldehyde:
Q.1) Calculate m/z values for each of the following:
(S-16, 2 Mark)
Q.2) Calculate m/z values for each of the following:
(S-16 & W-17, 2 Mark)
O +
C-H
.
m/e = 106
[C6H5-C-H]
O
+
.
m/z = 106](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-63-320.jpg)

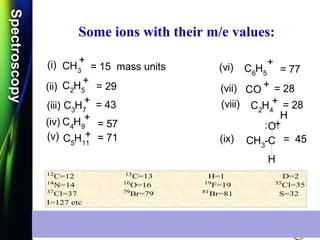
![SpectroscopySpectroscopy
Some Important example on
Mass Spectroscopy:
Q.1) Write the molecular ions of the following
compounds and calculate the m/e values: (S-
12, S-14 & W-14, 2 Mark)
CH3-NH2
Ans: Fragments of m/e = 31, 30 & 28
+.CH3NH2 + e
- 10 - 70 eV
[ CH3NH2 ]
m/e = 31OR
+.
CH3 NH2 + e
- 10 - 70 eV
[ CH3 NH2 ]
m/e = 31
..](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-66-320.jpg)
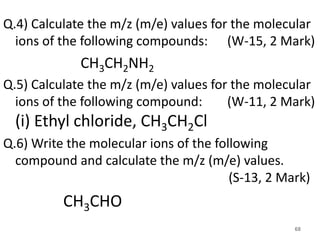
![Q.2) Calculate the m/z (m/e) values for the
following ion in mass spectroscopy:
(S-15 &S-19, 2 Mark)
• Ans: m/e = 31
Q.3) Calculate the m/z (m/e) values for each of the
particles. (S-11, S-13 & S-14, 2 -4 Mark)
+.
[ CH3NH2 ]
(i) CH2=NH2 & (ii) CH3C O
+ .+ .](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-67-320.jpg)
![Q.7) Calculate the m/z (m/e) values for the
following ion in mass spectroscopy:
[(CH3)2CH]+ (S-15 & S-19, 2 Mark)
Q.8) Calculate the m/z (m/e) values for the
following ion in mass spectroscopy:
(S-16, 2 Mark)
69
+
CH3
CH3
CH-NH2
[ [](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-69-320.jpg)
![Q.9) Calculate the m/z (m/e) values for the
following molecular ion in mass spectroscopy:
(W-16 & S-19, 2 Mark)
70
[C6H5-CH3]](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-70-320.jpg)

(ii) [CH3COOH]](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-71-320.jpg)
![Last Problem:
72
.
CH3CH2OH
[CH3CH2OH]
2e
2e
O
C
H3C CH3
O
H3C CH3
2e
e.g.-1)
e.g.-2)
e.g.-3)
m/e=78
m/e=46
m/e=58
+ e-
+ e-
+ e- 10 - 70 eV
10 - 70 eV
10 - 70 eV](https://image.slidesharecdn.com/massspectroscopybydr-200306190802/85/Mass-spectroscopy-by-dr-pramod-r-padole-72-320.jpg)