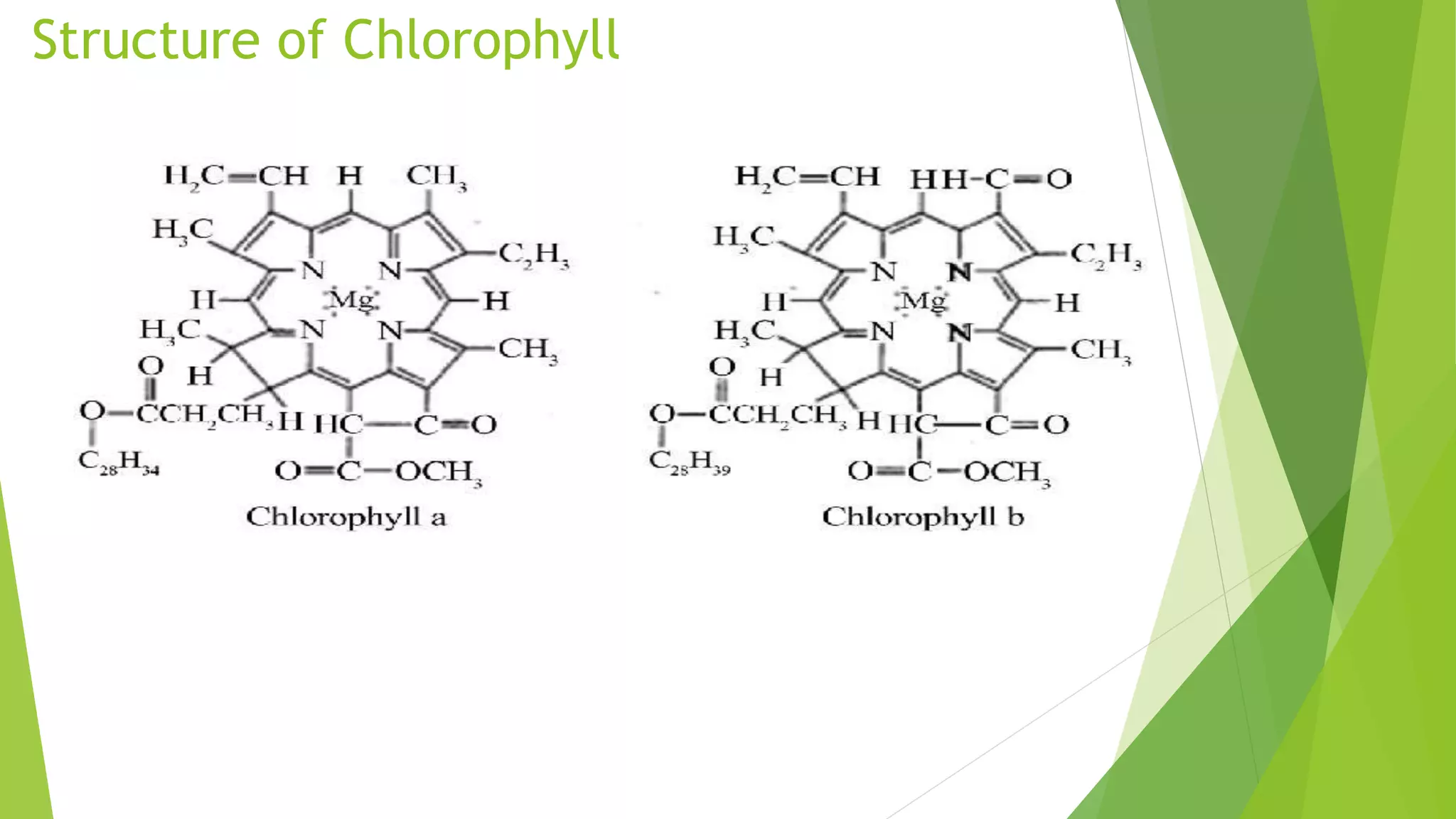
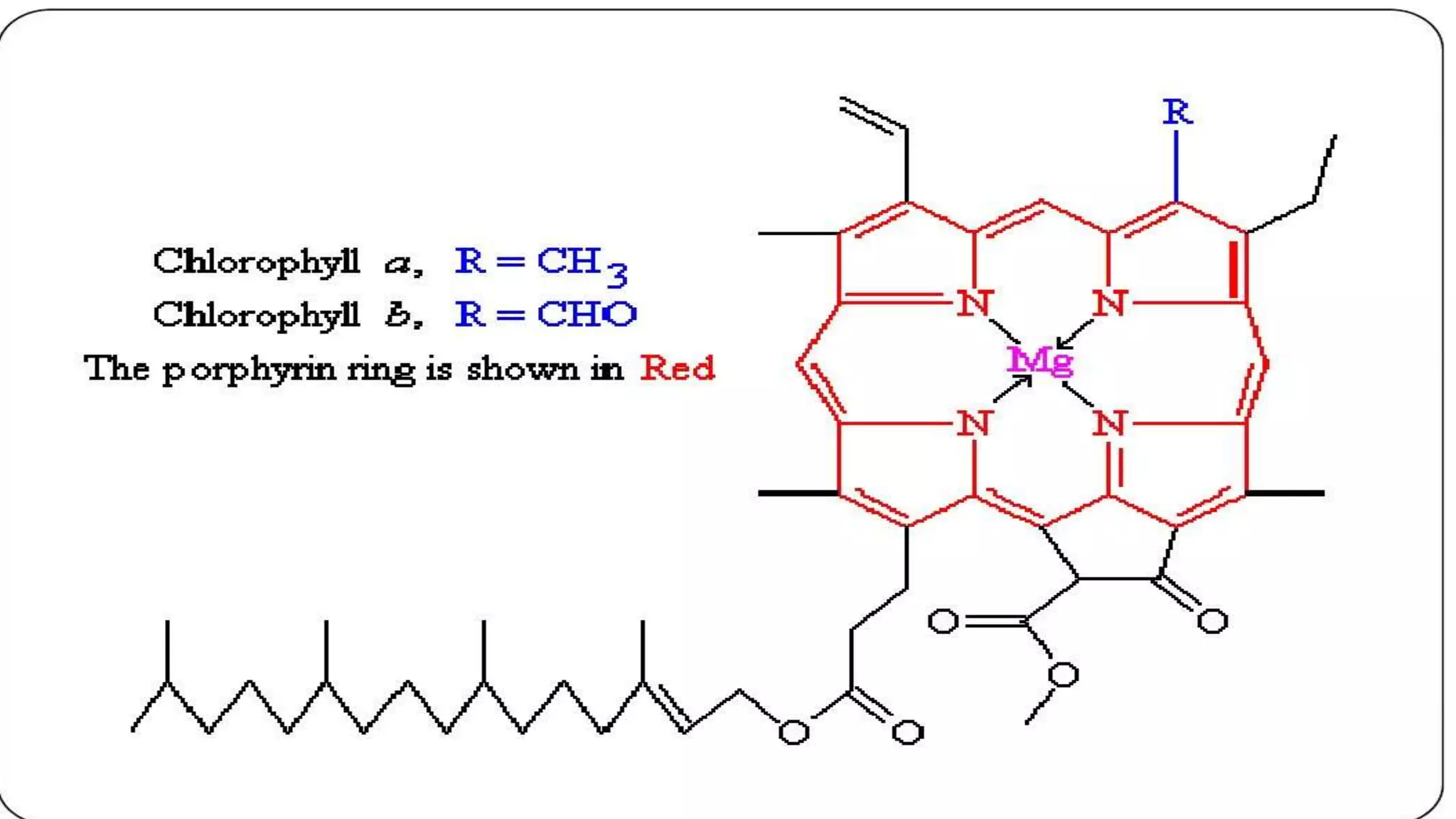
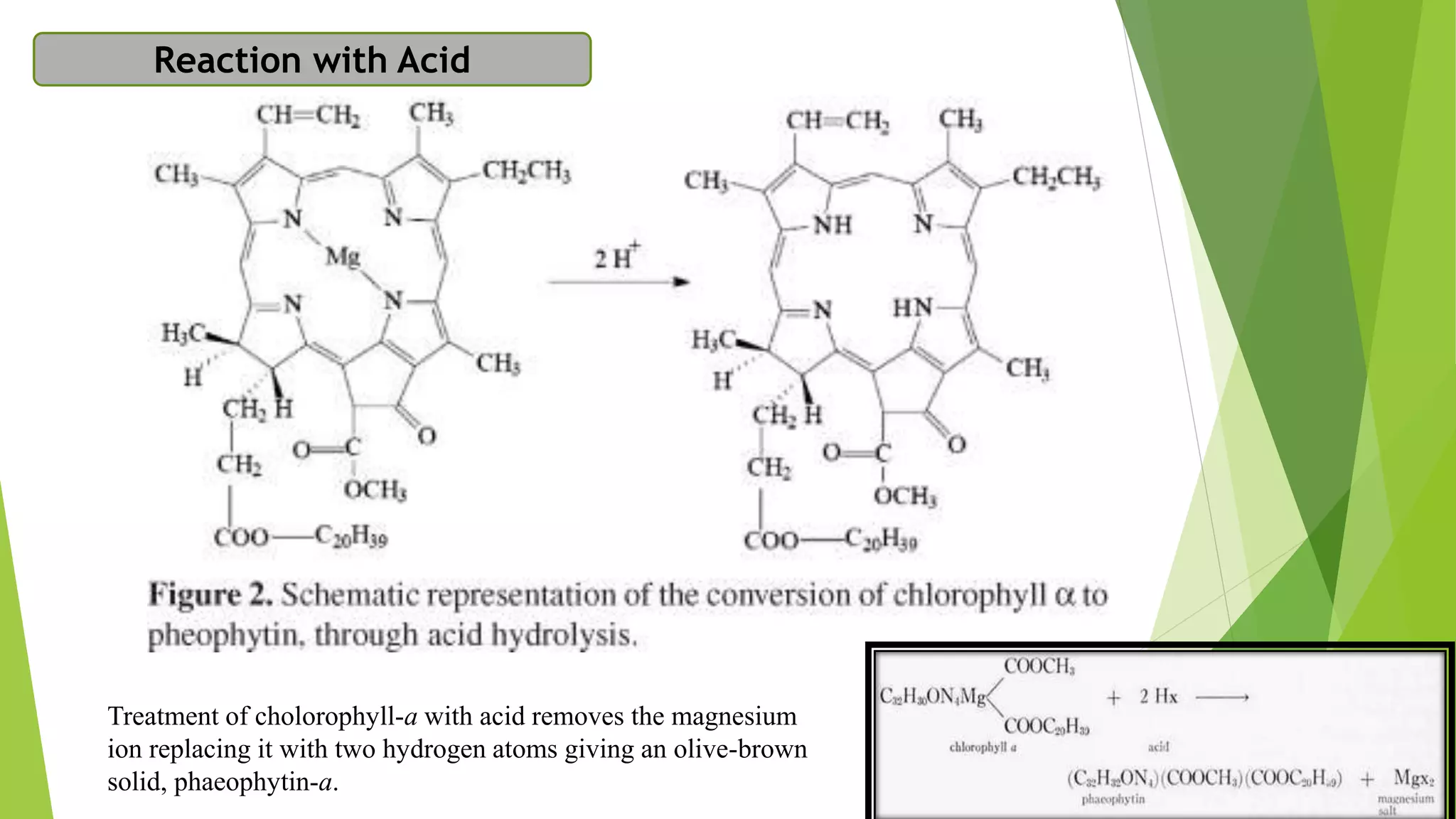
Chlorophyll is the green pigment found in plants that is essential for photosynthesis. It has a porphyrin ring structure with a magnesium ion at the center. There are different types of chlorophyll including chlorophyll a, b, c, d, and e. Chlorophyll a is the most abundant and important for photosynthesis. It is located within chloroplasts in plants and absorbs light energy to power the photosynthetic reaction that converts carbon dioxide and water into carbohydrates. Chlorophyll plays a key role in photosynthesis by using the absorbed light energy to transfer electrons that are used to reduce carbon dioxide. Chlorophyll can be extracted from plants using detergents and has many health benefits when