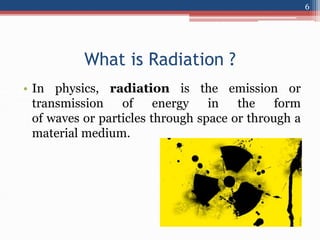
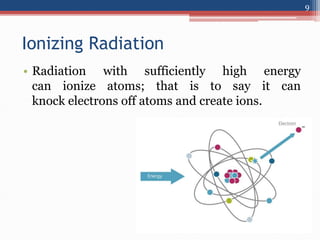
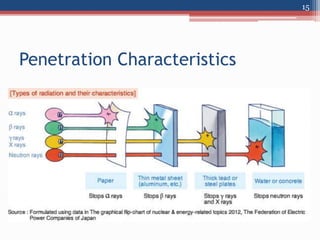
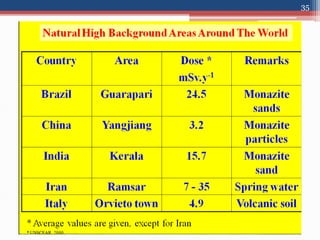
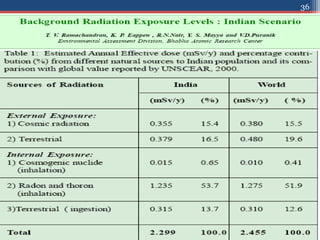
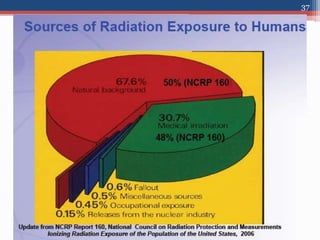
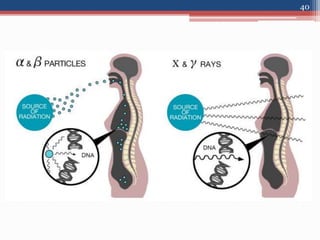
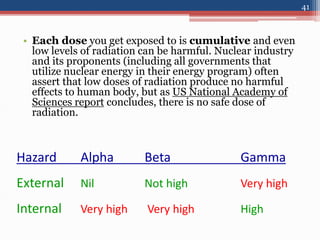
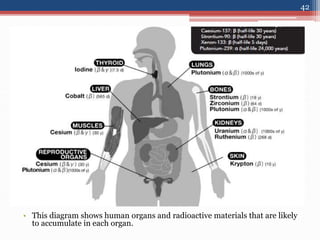
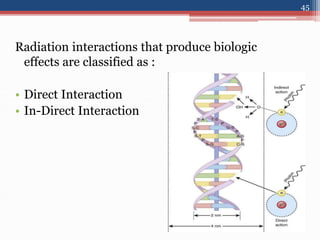
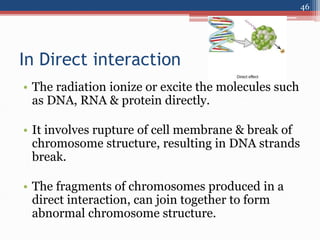
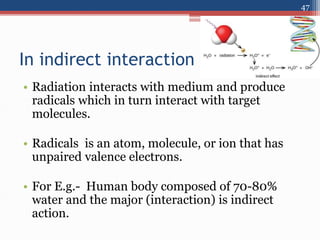
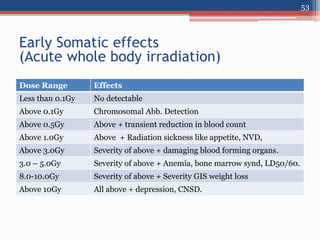
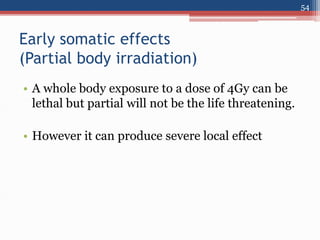
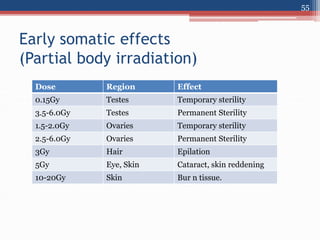
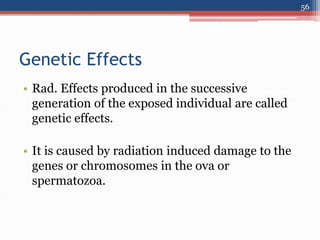
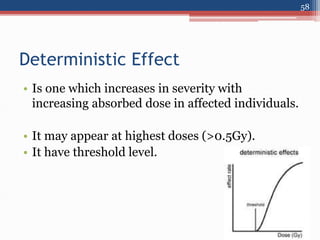
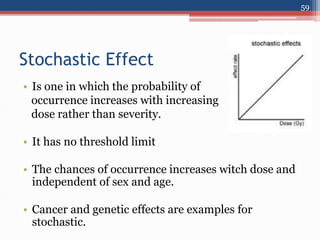
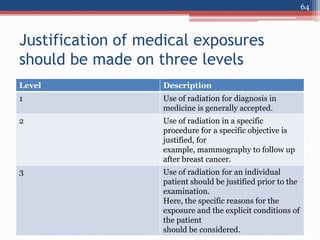
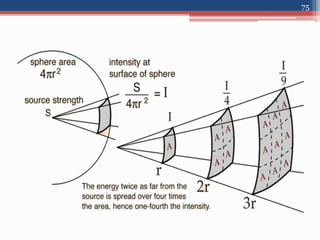
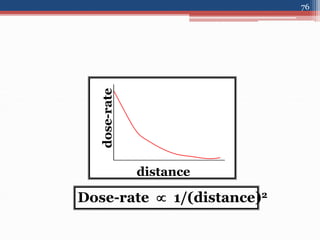
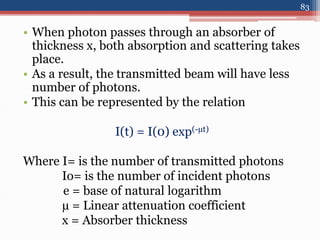
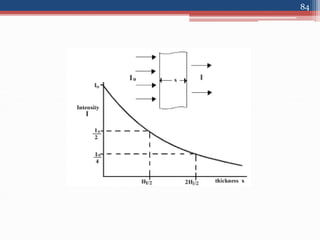
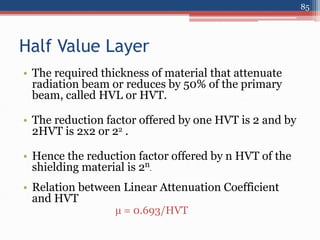
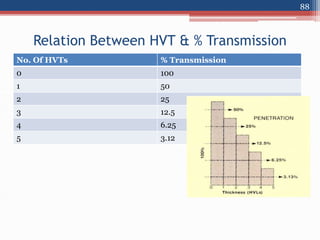
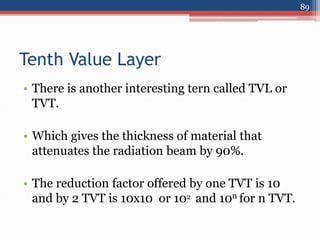
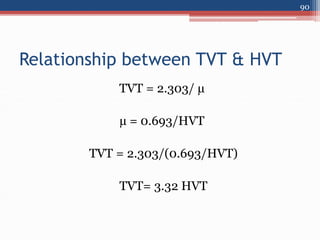
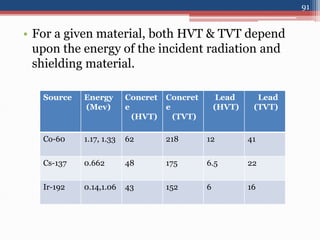
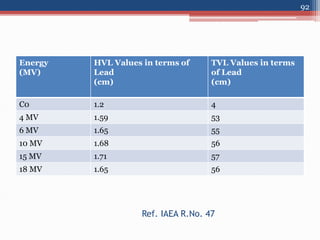
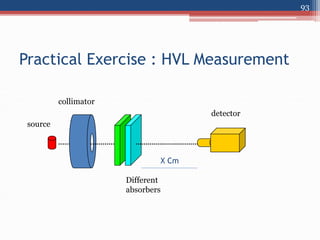
Radiation can be categorized as ionizing or non-ionizing. Ionizing radiation includes alpha, beta, gamma, and neutron radiation, which can be harmful in high doses. Natural background radiation comes from cosmic rays, terrestrial sources like uranium and thorium in soil, and inhaling or ingesting naturally occurring radioactive materials. The largest sources of natural background radiation are radon inhalation and terrestrial sources. Artificial sources include residual radiation from past atmospheric nuclear weapons testing. The document discusses the various types of radiation and their sources to provide an understanding of radiation hazards, evaluation, and safety.