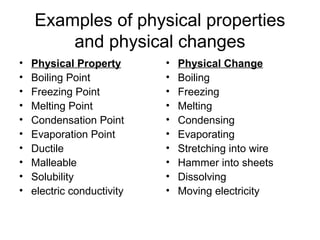
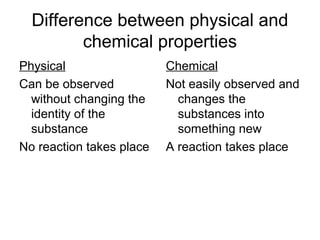
This document discusses the differences between physical and chemical properties of matter. Physical properties can be observed or measured without changing the identity of the substance, such as boiling point, density, state, and color. Physical changes alter the substance's form but not its composition, like melting, freezing, or bending. Chemical properties describe a substance's ability to undergo chemical reactions, for example flammability or reactivity. Chemical changes result in one or more new substances forming through chemical reactions. The identity of the original substance is changed in a chemical change.