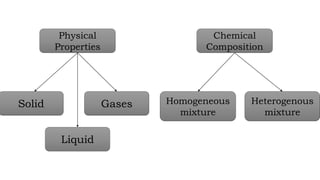
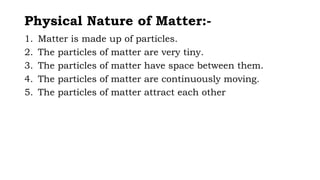
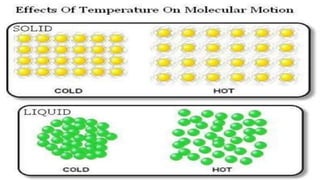
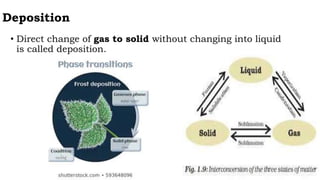
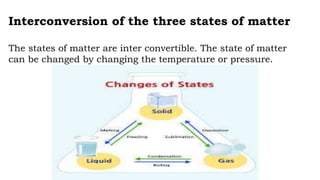
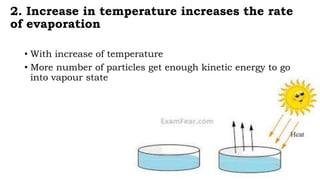
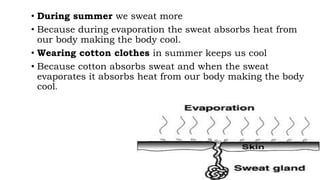
Chapter 1 discusses the nature of matter, defining it as anything that occupies space and has mass. Matter is classified into solid, liquid, and gas states based on particle arrangement, movement, and attraction forces, and includes experiments to illustrate concepts like particle distribution, diffusion, and changes in state due to temperature and pressure. The chapter also covers concepts like melting, boiling, evaporation, and factors affecting these processes, emphasizing the kinetic behavior of particles and latent heat in phase transitions.