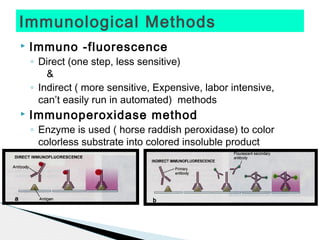
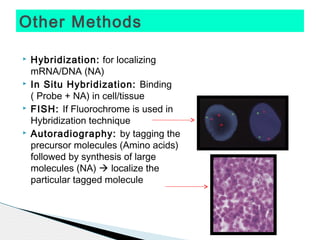
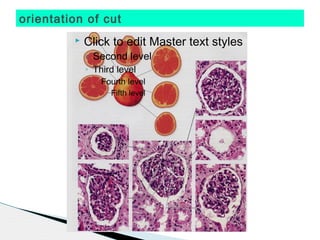
* Treponema pallidum causes syphilis.
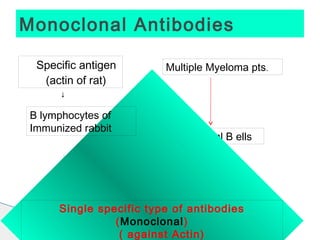
* A highly aneuploid tumor indicates genomic instability which is associated with increased aggressiveness and poor prognosis.
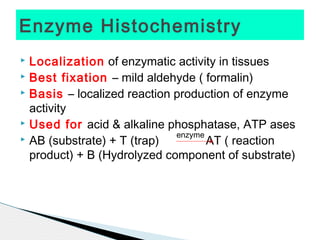
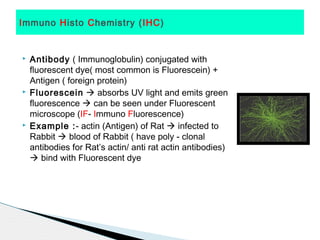
* Amyloid protein showing apple green birefringence under polarized light microscopy has clinical significance as it indicates amyloidosis.
* The signs ±, ± ± indicate the type of birefringence shown by substances under polarized light microscopy. ± indicates negative birefringence shown by uric acid while ± ± indicates positive birefringence shown by calcium pyrophosphate.
* Osmium tetroxide reacts with phospholipids in cell membranes, making them electron dense and visible under electron microscopy. This helps in better structural visualization of cells