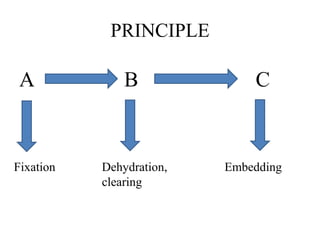
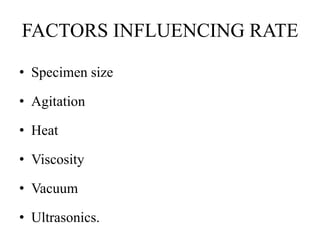
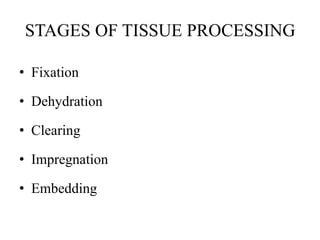
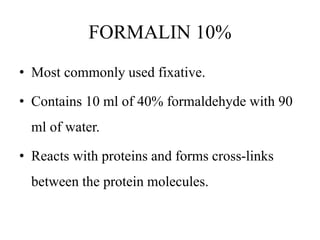
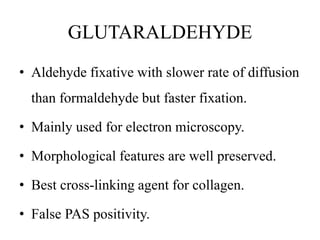
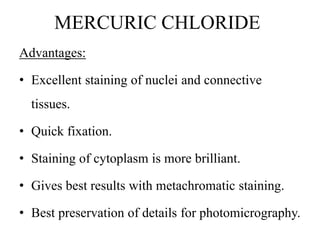
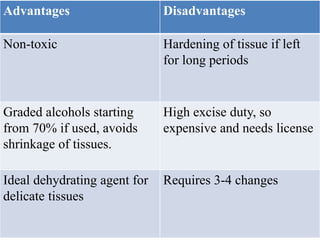
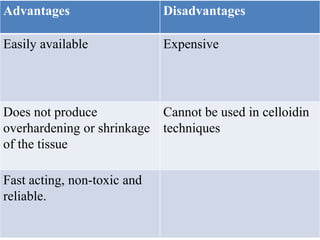
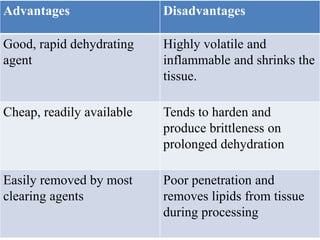
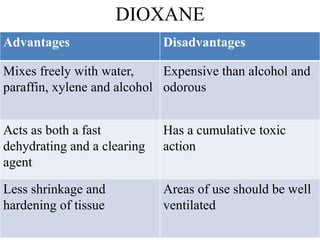
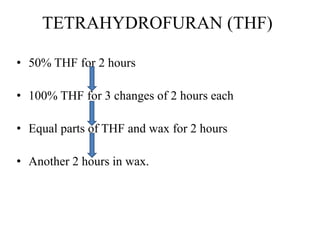
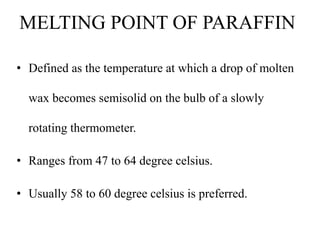
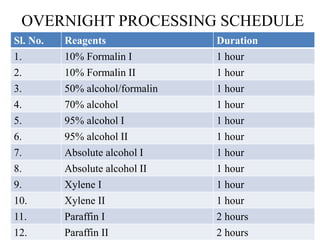
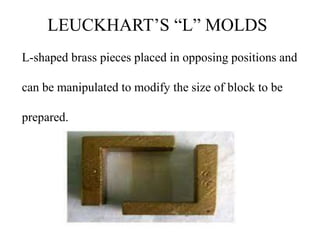
This document discusses the process of tissue processing, which involves fixing, dehydrating, clearing, and embedding tissue samples in paraffin wax to allow for thin sectioning. The key stages are fixation using chemicals like formalin to preserve tissue structure, dehydration using increasing concentrations of alcohol, clearing using solvents like xylene to make tissues transparent, and embedding in paraffin wax for sectioning. Automated and manual methods are described. Tissue microarrays allow evaluation of multiple tissue samples on a single slide by arranging small cores in a recipient paraffin block.