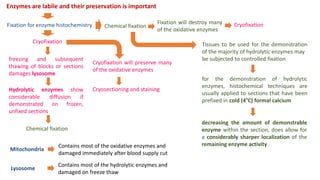
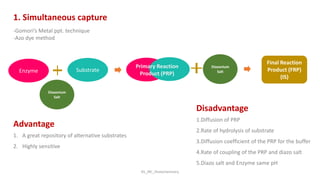
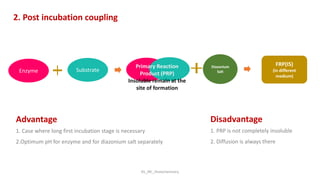
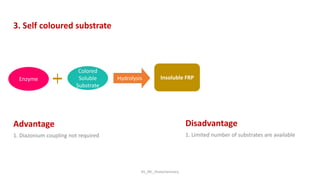
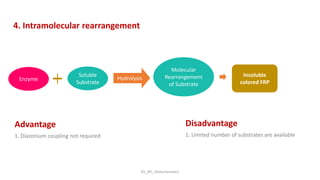
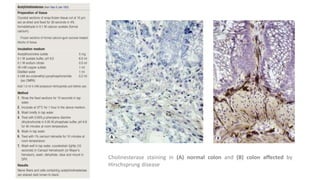
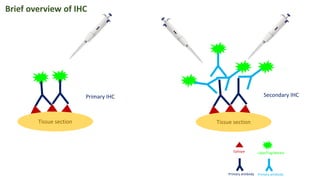
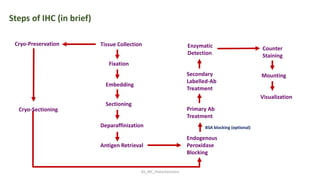
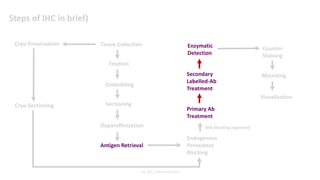
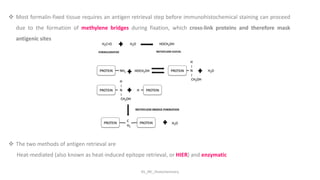
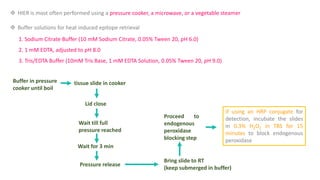
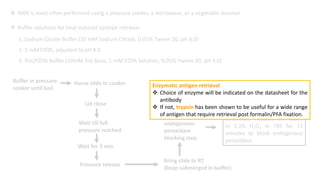
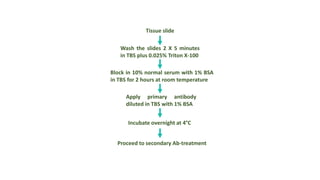
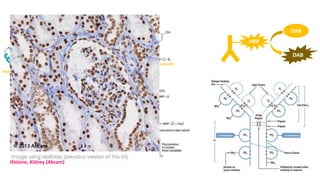
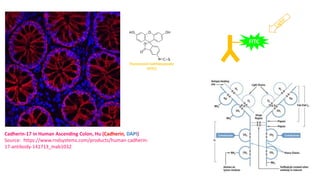
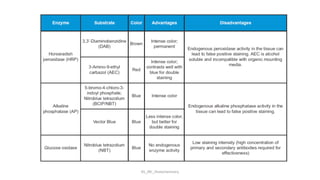
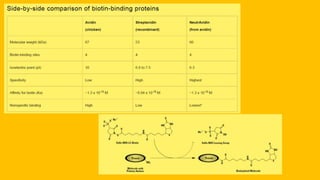
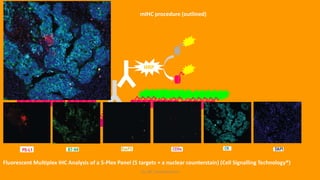
Histochemistry is a science that combines techniques of biochemistry and histology to study the chemical composition of tissues. Enzyme histochemistry techniques are applied to tissue sections that have undergone controlled chemical fixation or cryofixation to preserve enzyme activity. Common applications include skeletal muscle biopsy to identify fiber types using enzymes like ATPase, and colonic biopsy to diagnose Hirschsprung's disease by detecting acetylcholinesterase in ganglion cells. Immunohistochemistry identifies cellular antigens using antigen-antibody binding and involves tissue collection, fixation, antigen retrieval, primary/secondary antibody incubation, detection such as HRP-DAB, and counterstaining.