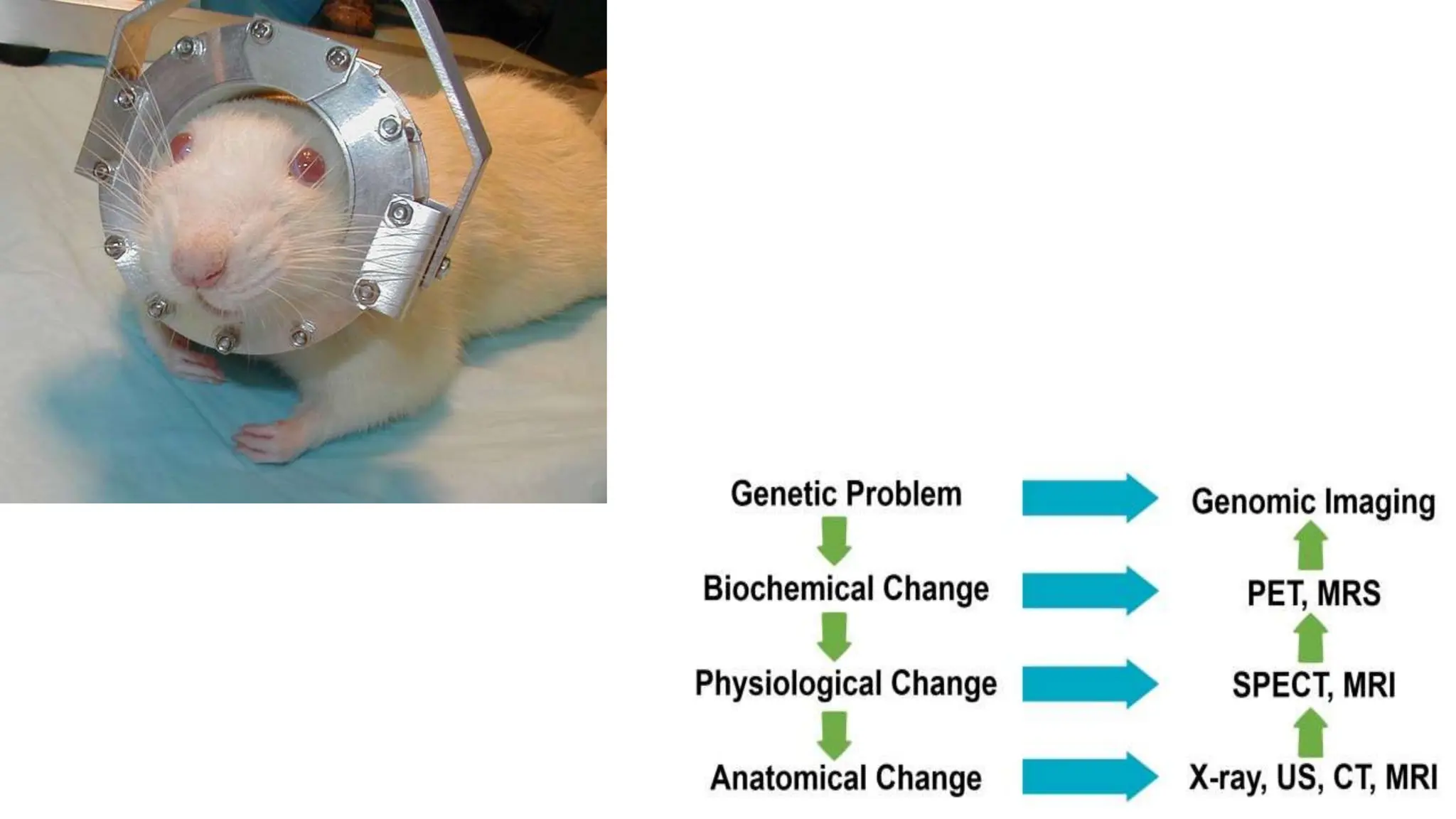
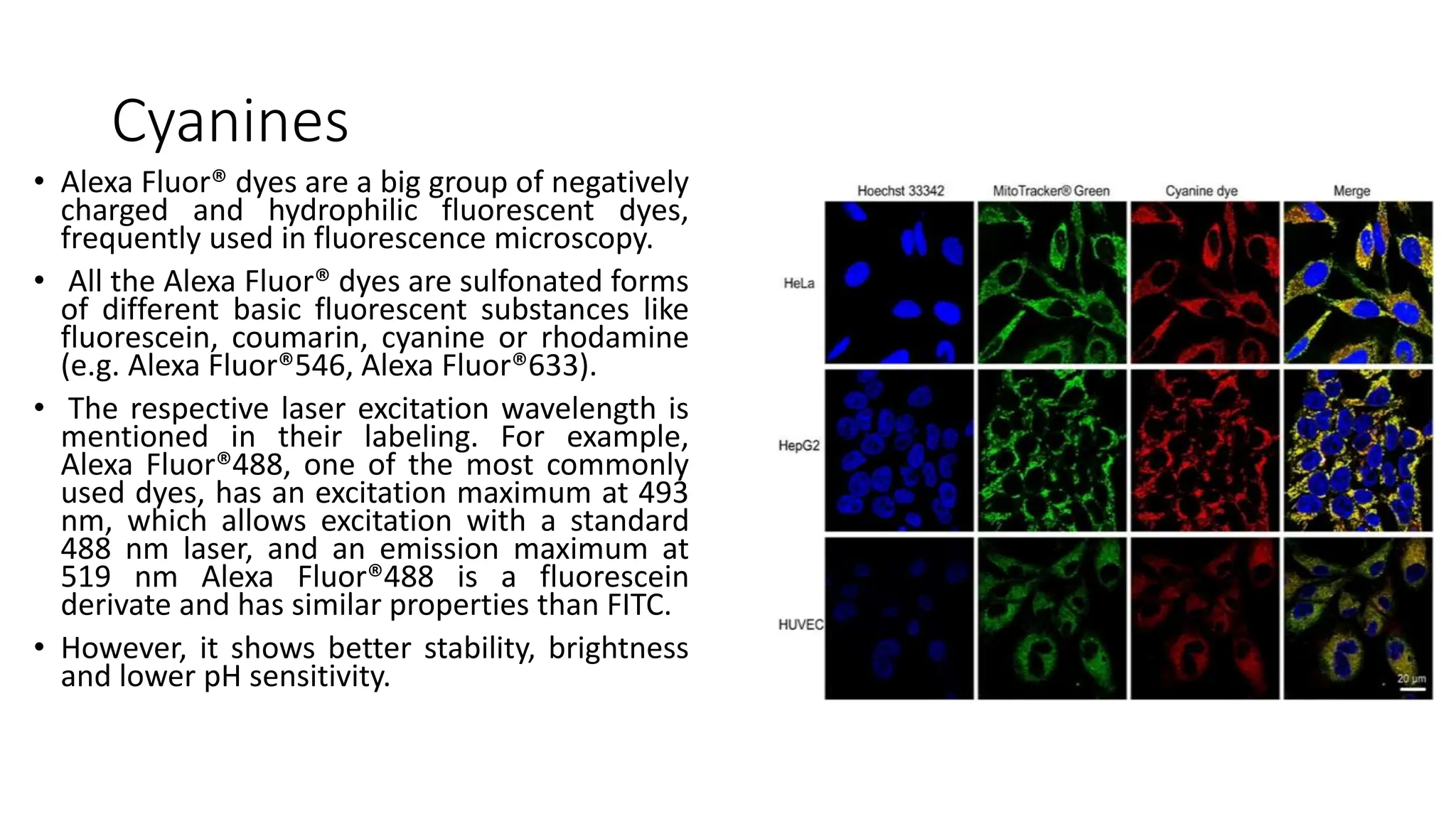
The document discusses various imaging techniques used in pharmacological research, including AI-powered applications and traditional microscopy. It highlights the significance of imaging tools in diagnosing medical conditions, tissue preparation methods, and the use of fluorescent agents for visualization. Additionally, it mentions specific applications in different medical fields, such as oncology and dermatology, and outlines the advancements in imaging technologies.