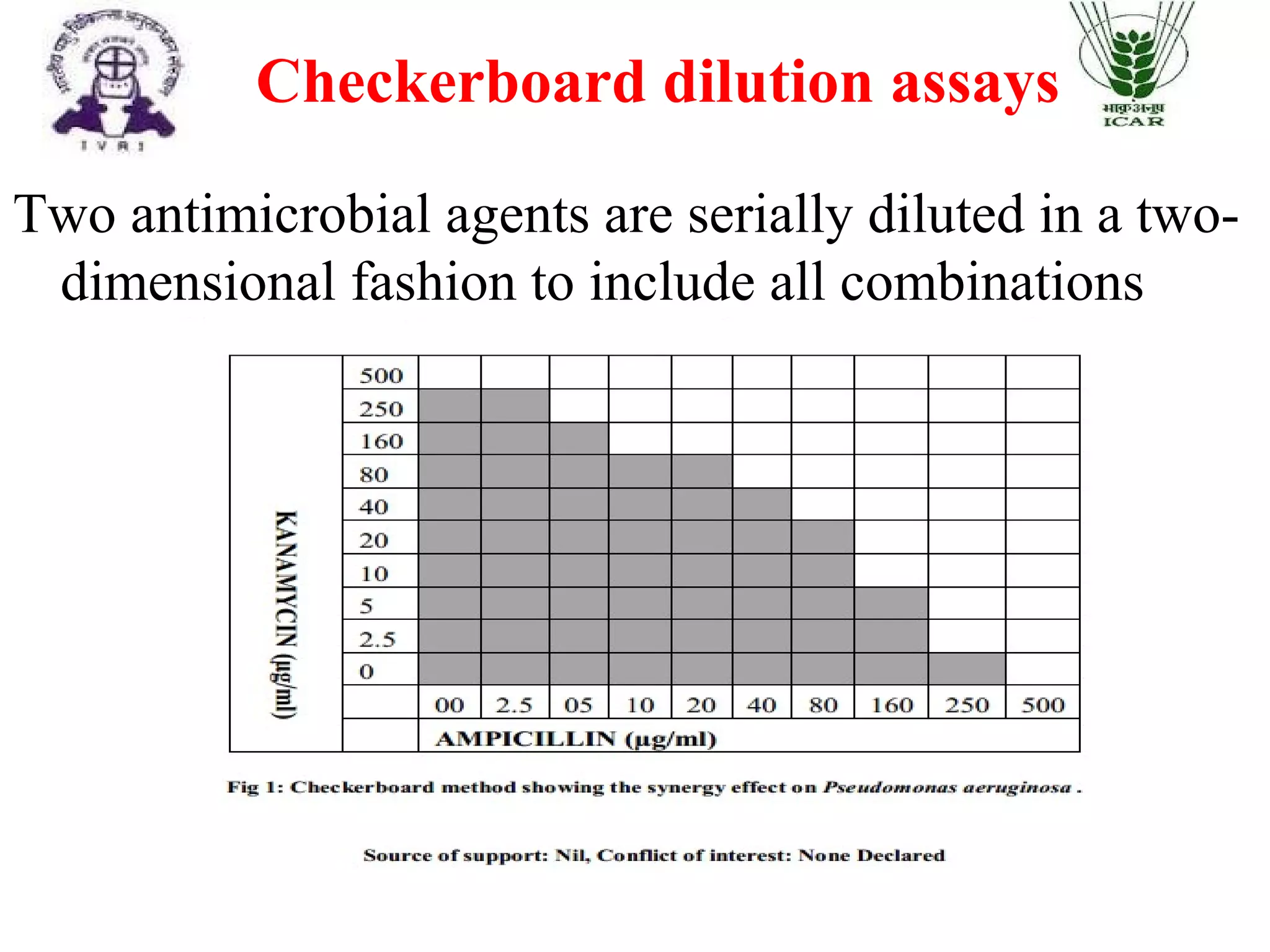
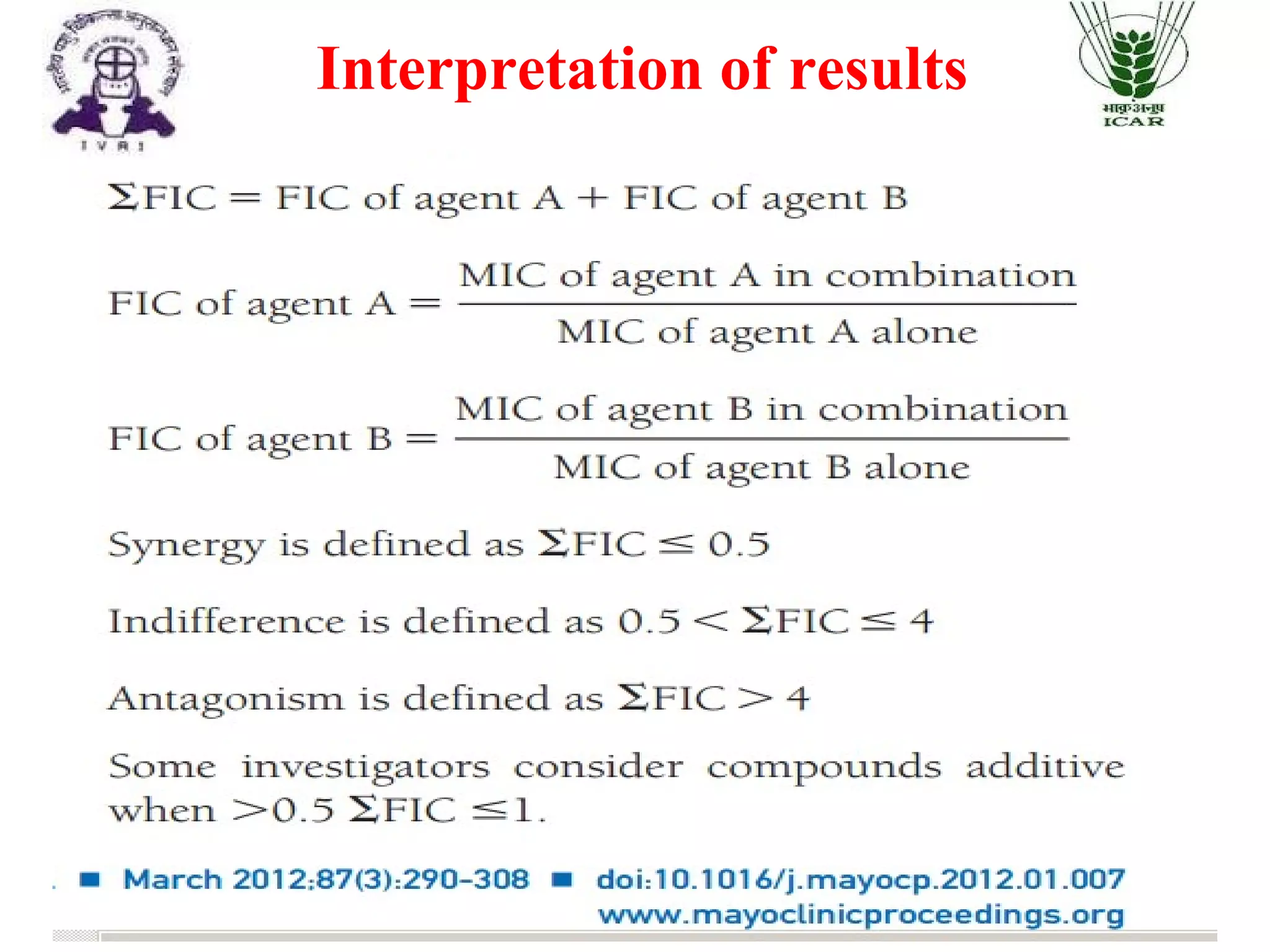
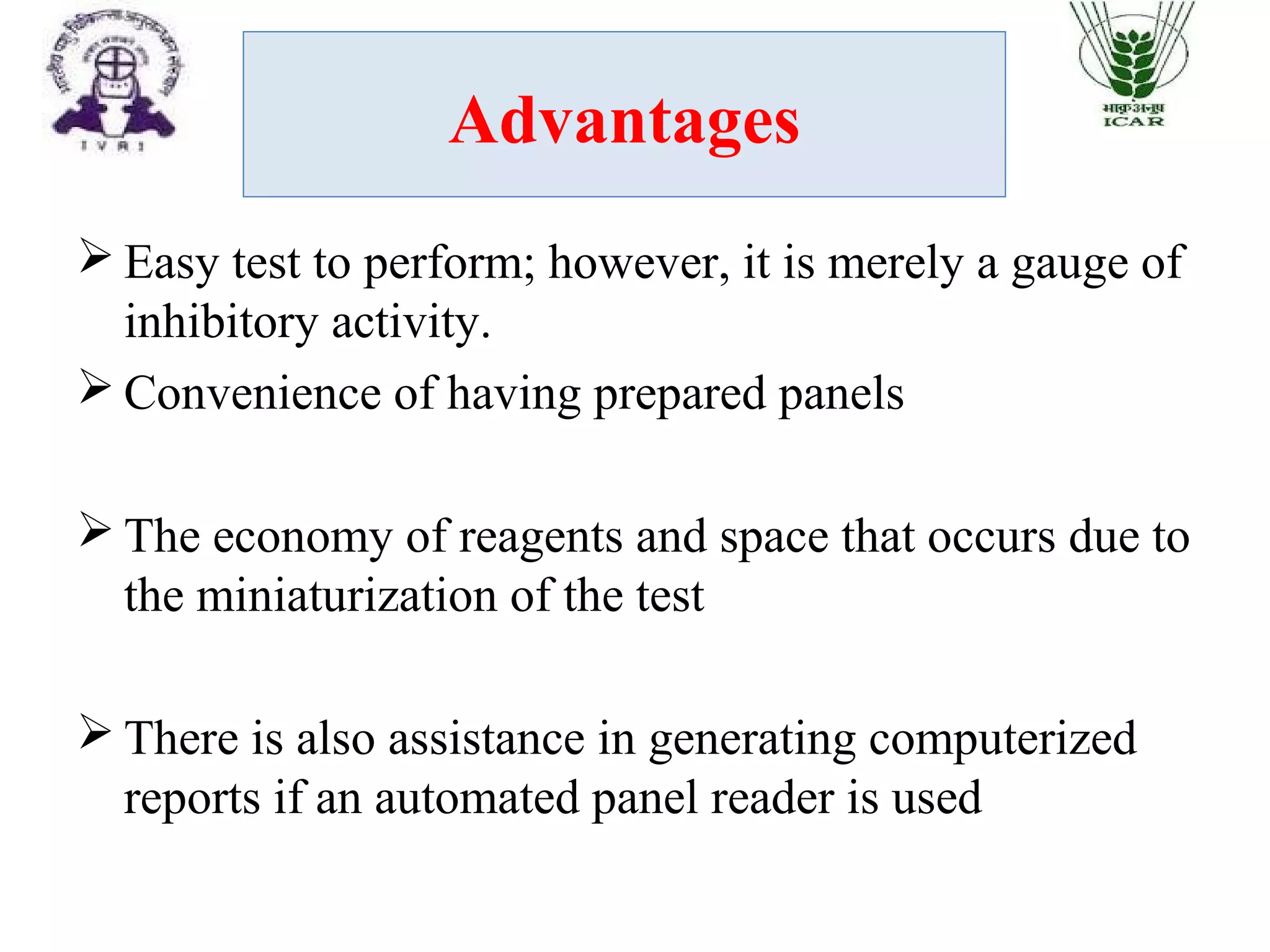
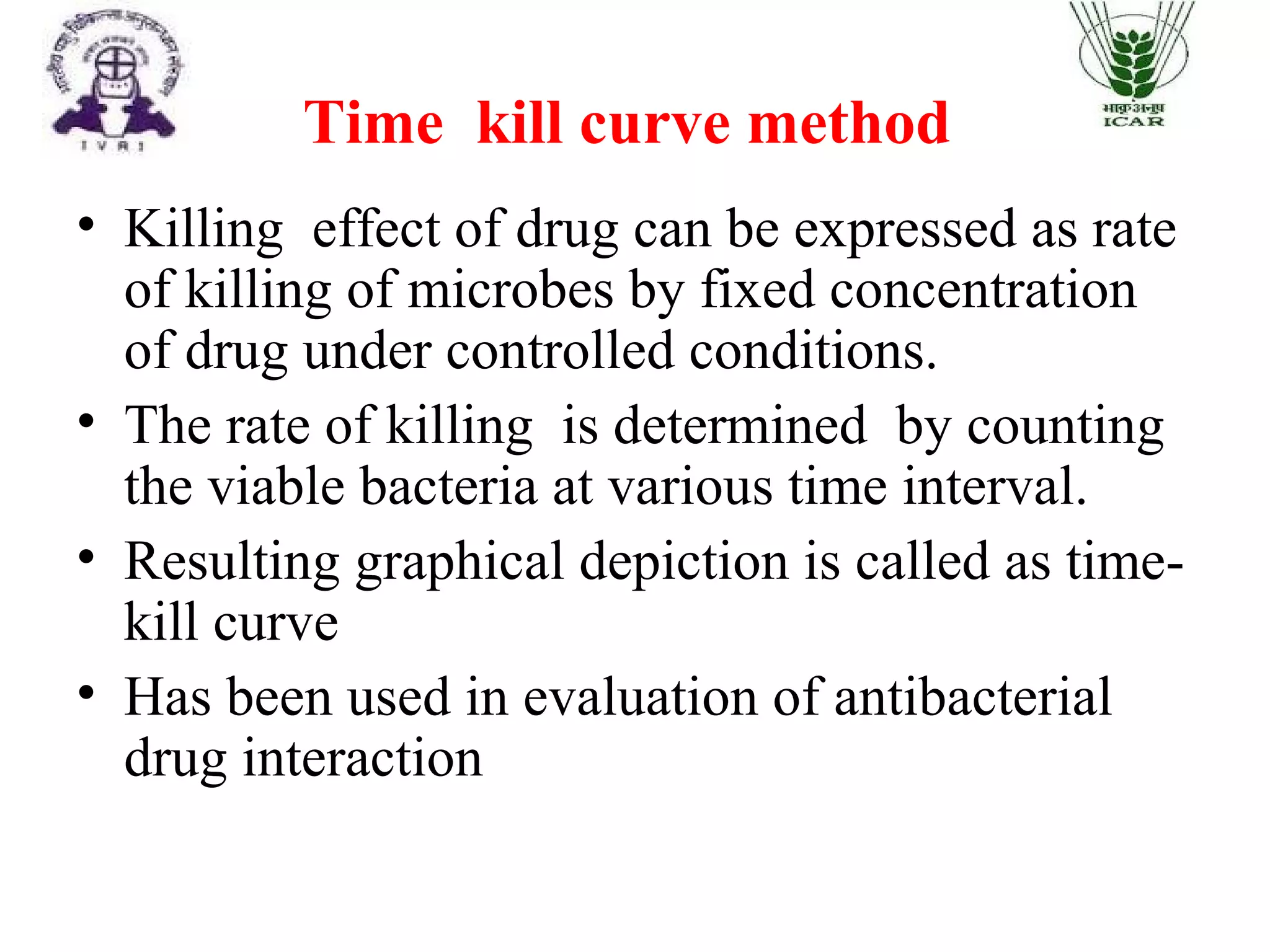
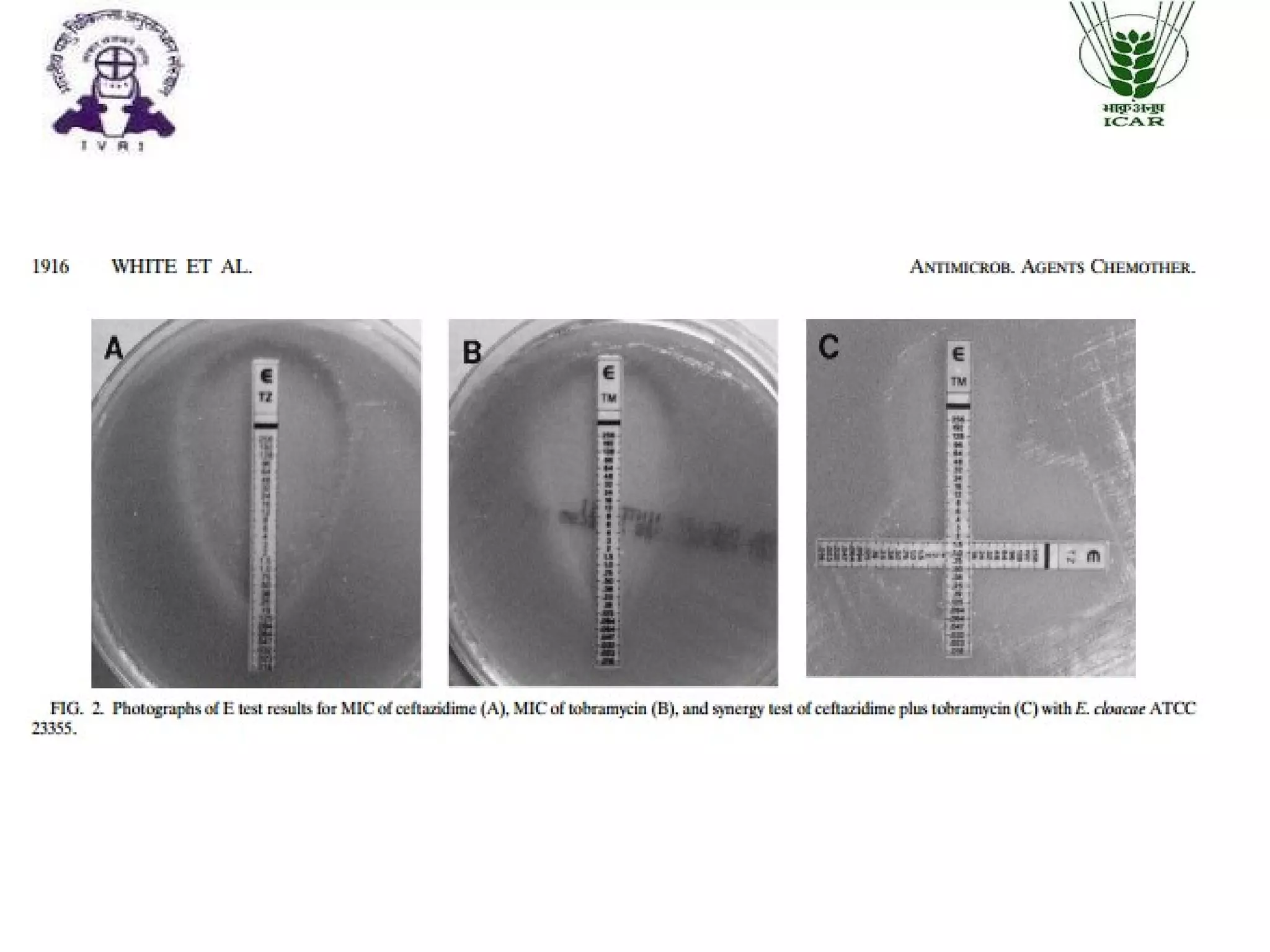
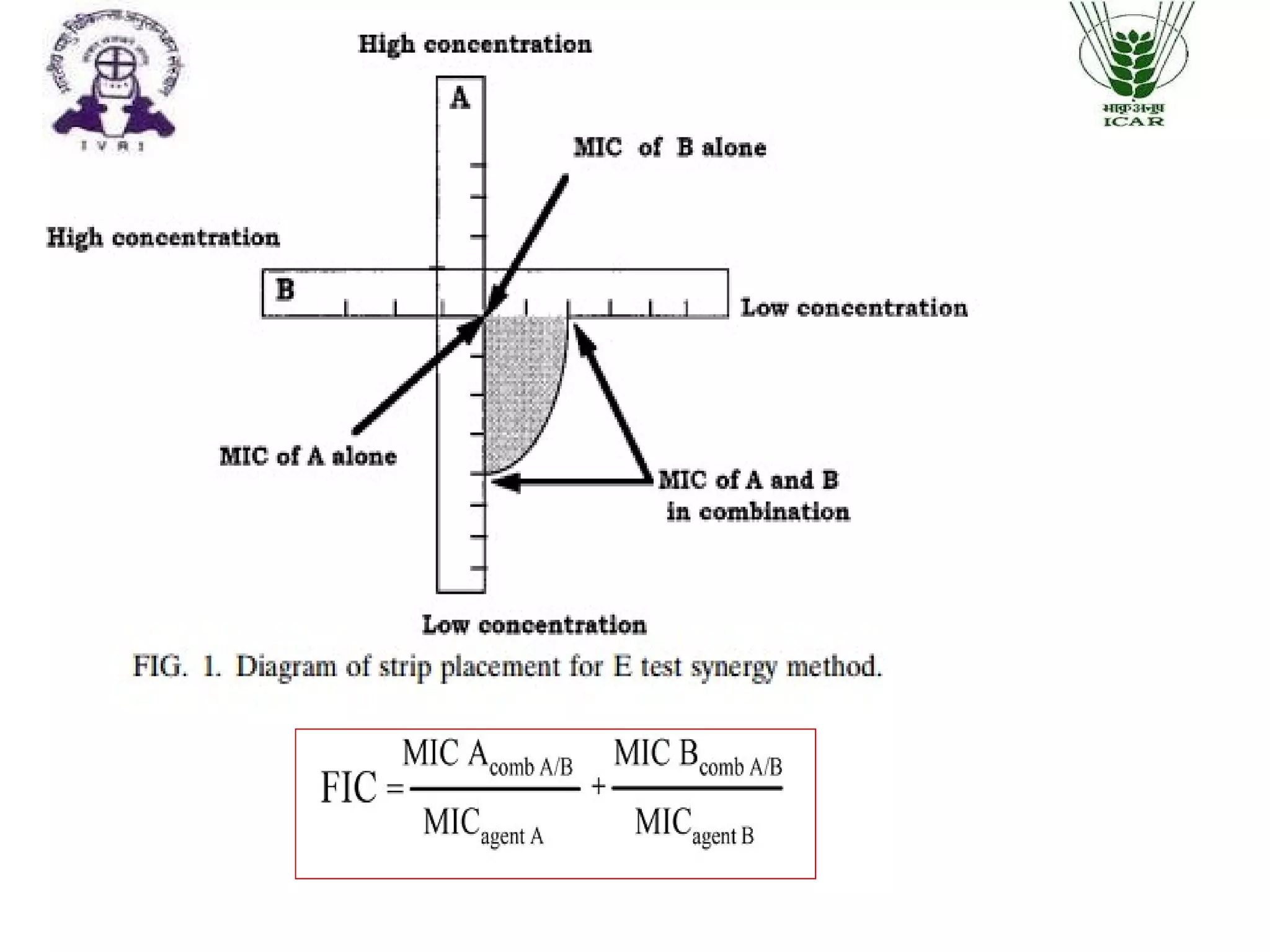
The document outlines methods for determining fractional inhibitory concentration (FIC) to assess drug interactions in combination therapies, highlighting the importance of synergistic, additive, and antagonistic effects. It details various synergy testing methods such as checkerboard dilution assays, time-kill curve methods, E tests, and multiple combination bactericidal testing (MCBT), along with their advantages and disadvantages. The primary objectives are to enhance antibacterial activity while minimizing the risk of resistance development and toxic effects associated with high drug doses.