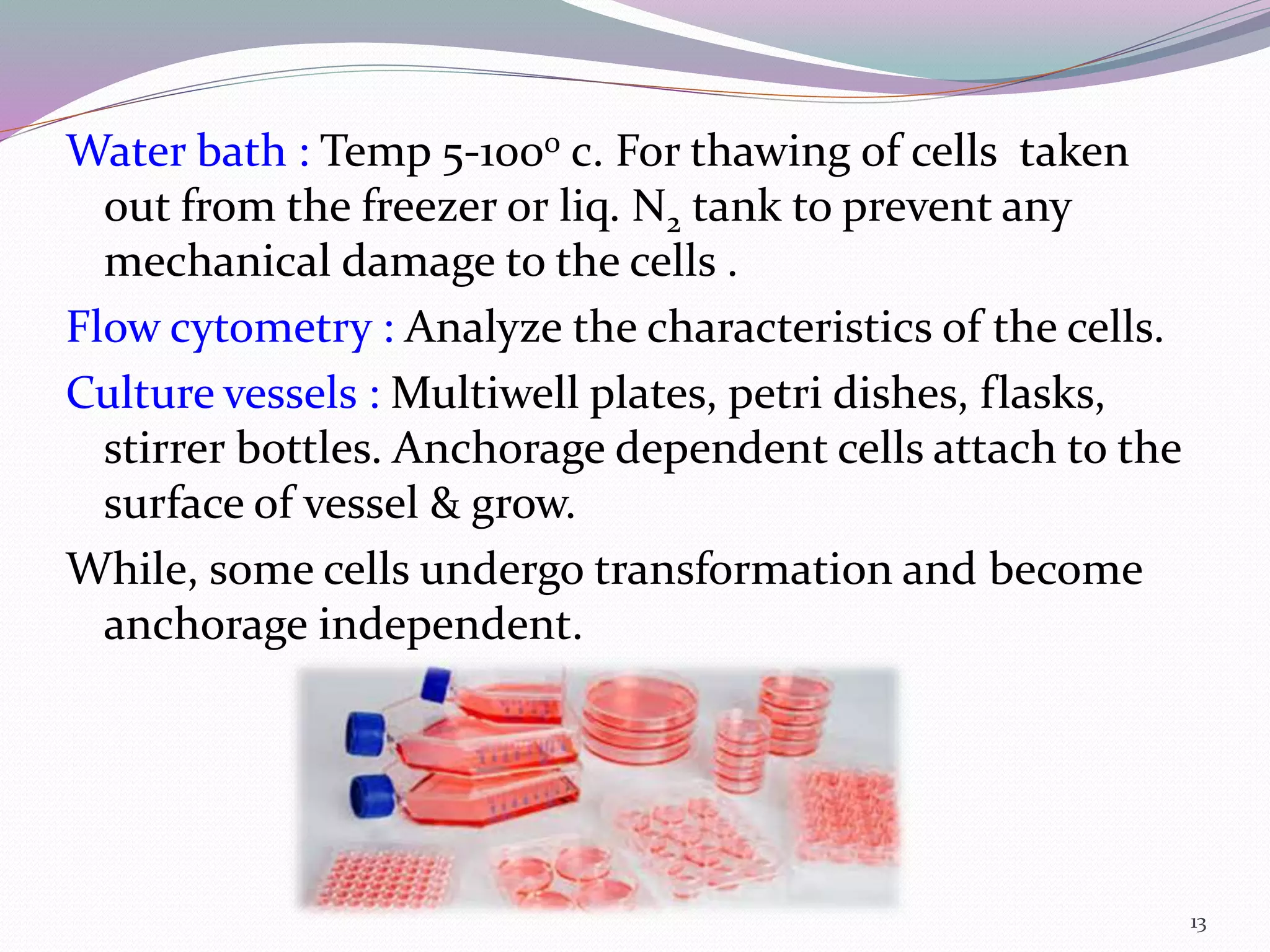
The document provides a comprehensive overview of cell culture, detailing its history, methodologies, and various terminologies such as primary culture and cell lines. It discusses key developments in technology, laboratory requirements, types of culture media, and the advantages and limitations of cell culture techniques. Additionally, the applications of cell culture in research, drug development, and biopharmaceutical production are outlined.