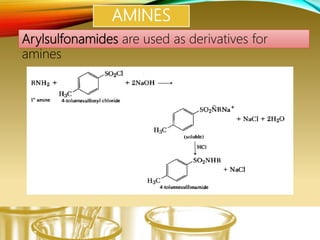
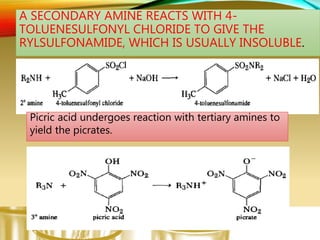
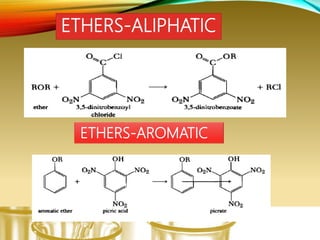
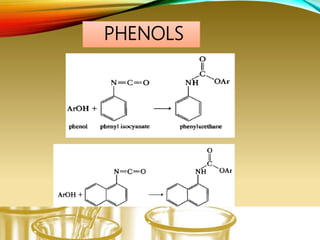
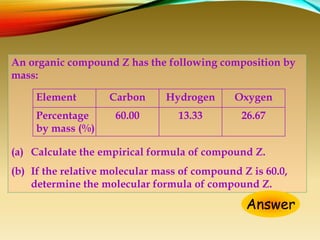
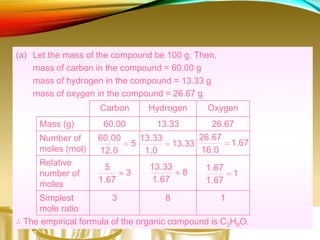
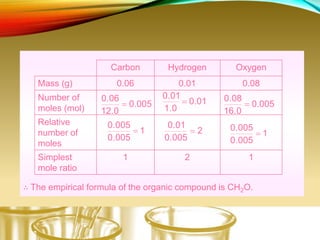
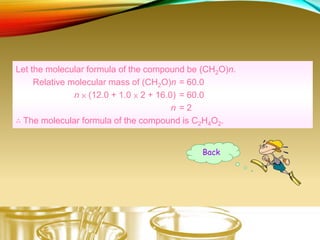
This document provides information on qualitative organic analysis including:
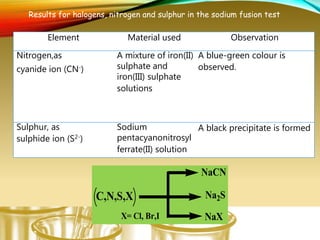
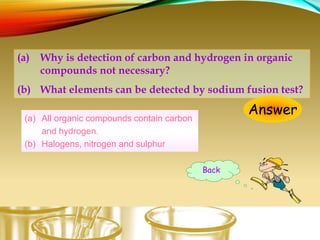
- Testing for elements like carbon, hydrogen, halogens, nitrogen, and sulfur using sodium fusion.
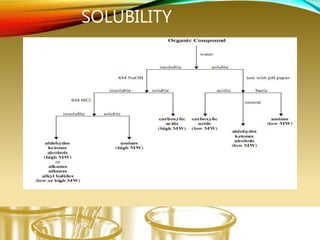
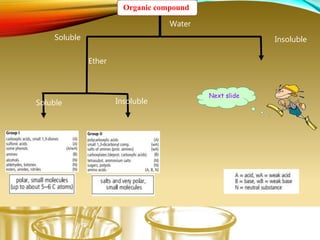
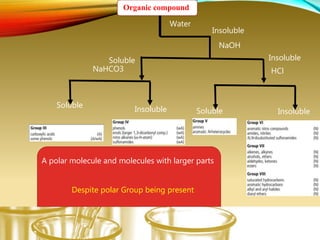
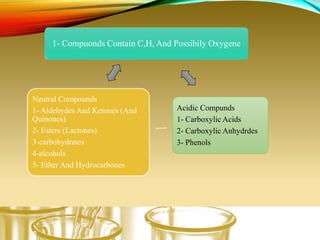
- Using physical properties and solubility tests to obtain structural information.
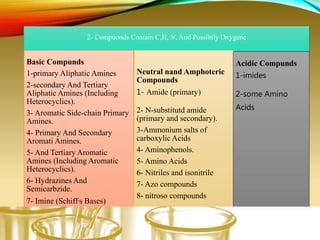
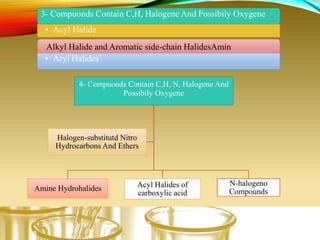
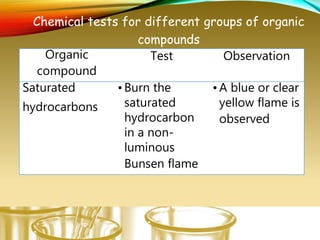
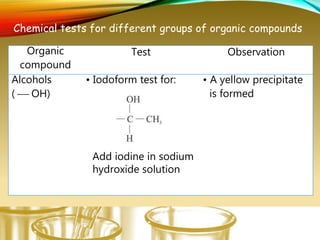
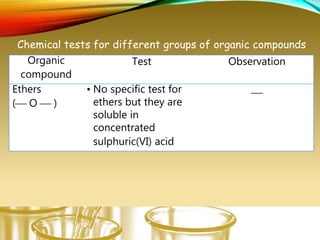
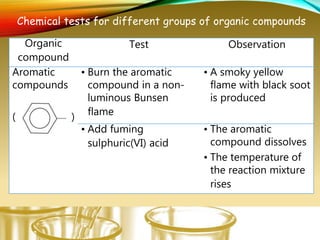
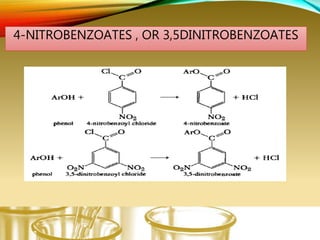
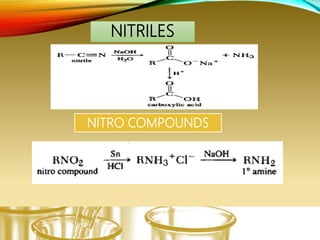
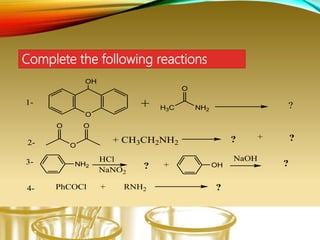
- Performing classification tests to identify functional groups like alkanes, alkenes, alcohols, aldehydes, ketones, carboxylic acids, esters, amines, ethers, phenols, nitriles, and nitro compounds.
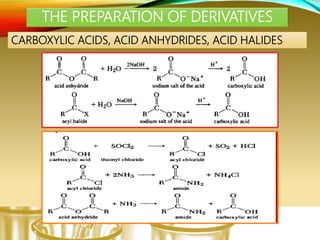
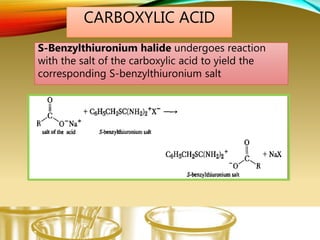
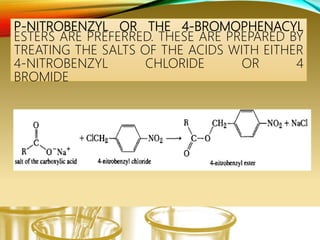
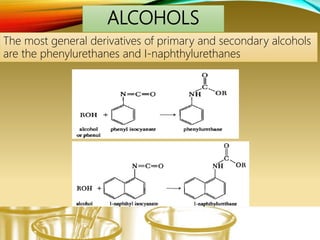
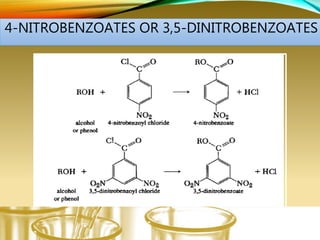
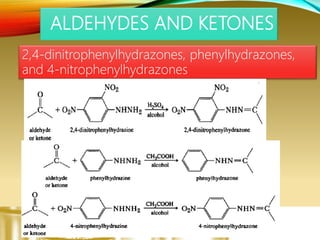
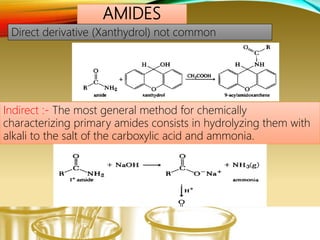
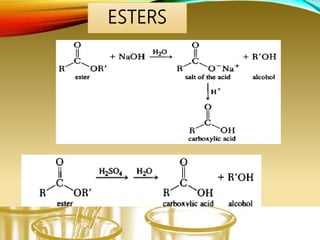
- Preparing characteristic derivatives to further identify organic compounds such as phenylurethanes for alcohols and arylsulfonamides for amines.