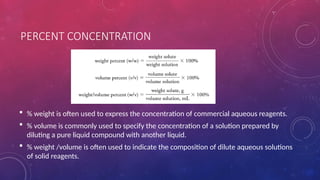
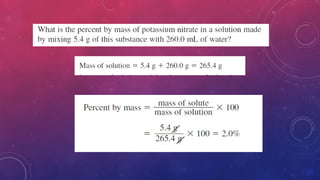
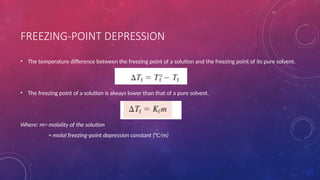
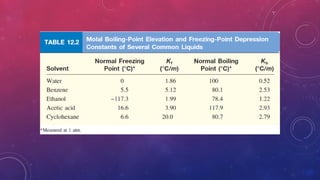
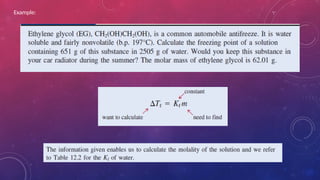
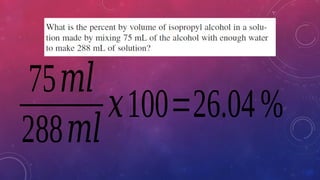The document discusses solutions, their types, and concentrations, defining terms such as solute, solvent, concentrated, and dilute solutions. It elaborates on methods to express concentration, including percent concentration, molarity, molality, and normality, along with examples for clarity. Additionally, it covers colligative properties like vapor pressure lowering and boiling point elevation, and provides relevant formulas and calculations.