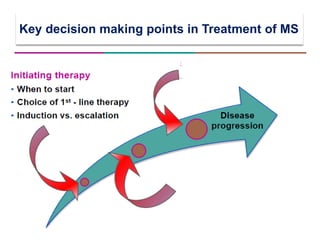
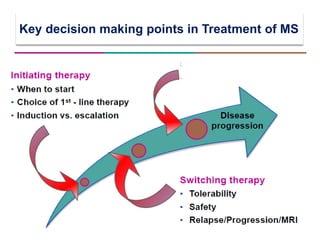
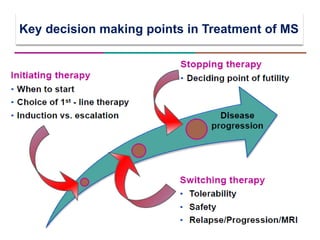
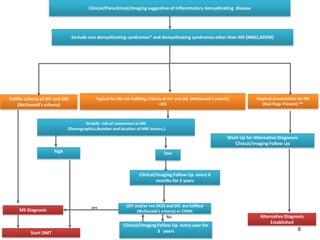
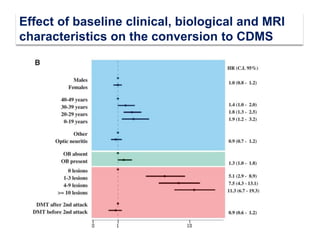
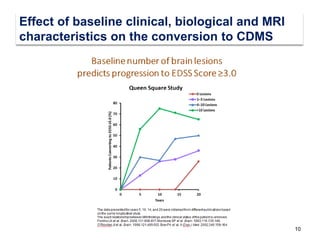
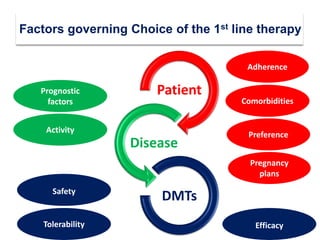
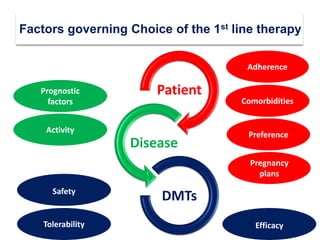
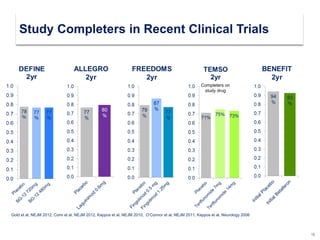
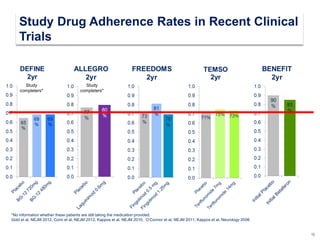
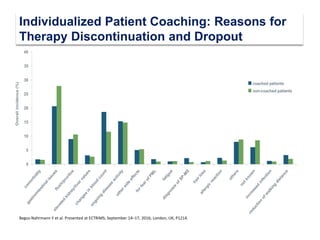
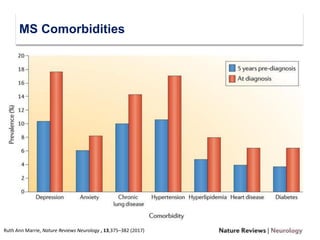
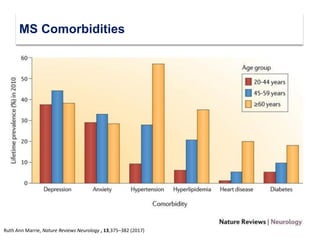
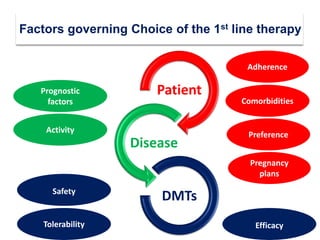
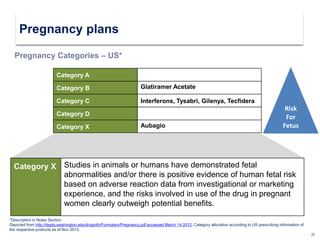
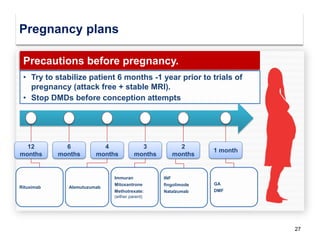
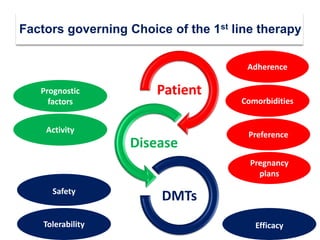
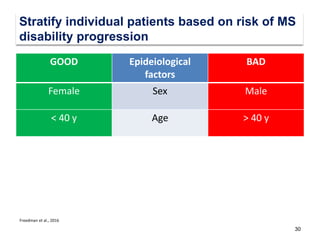
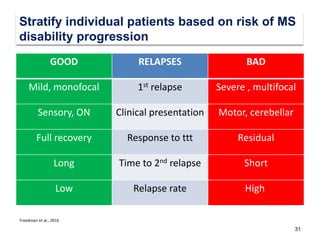
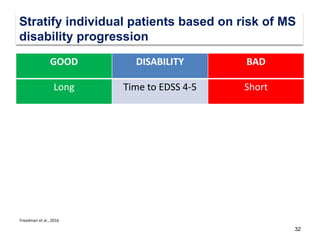
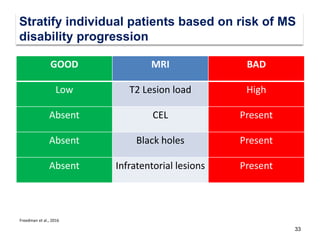
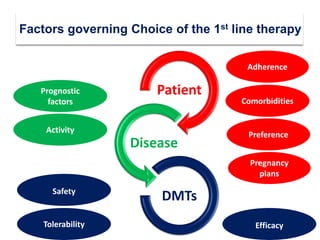
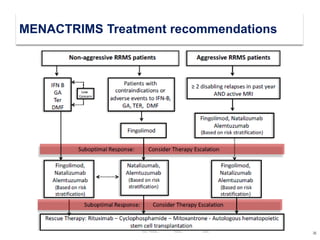
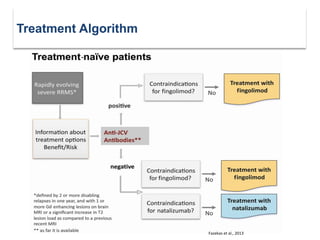
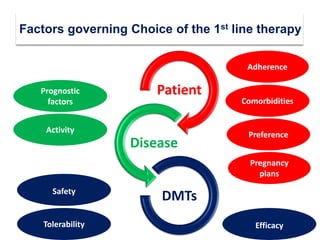
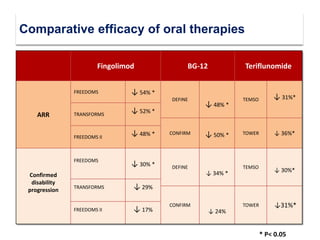
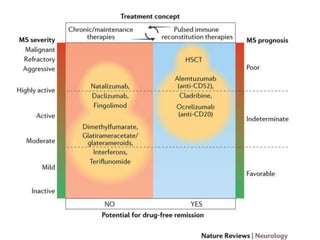
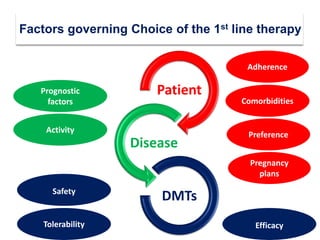
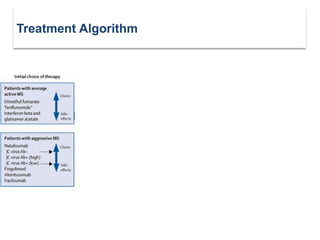
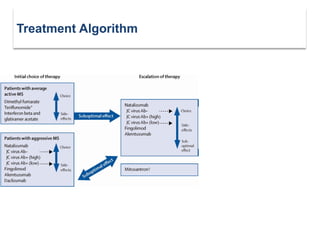
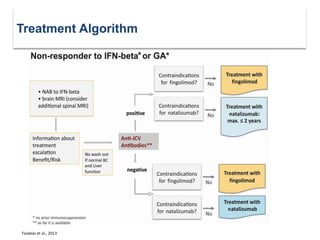
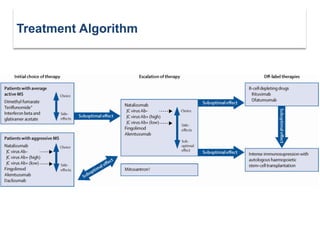
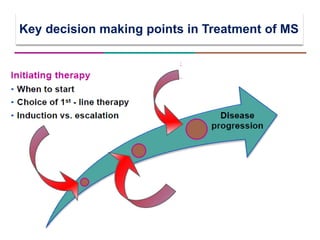
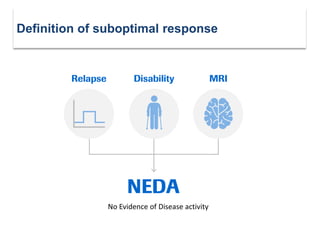
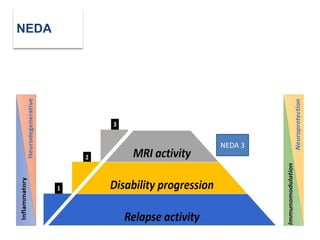
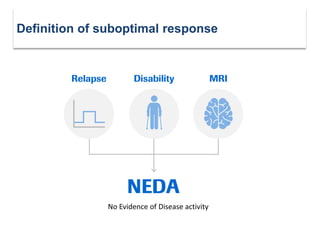
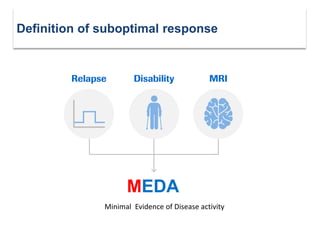
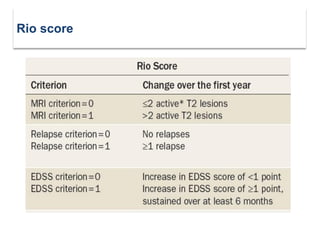
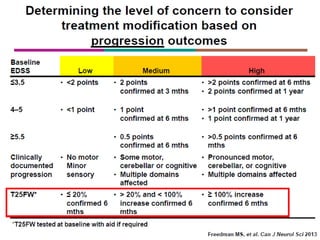
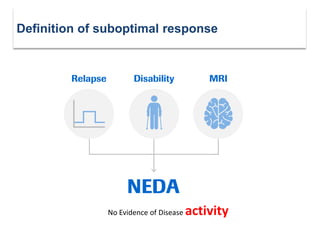
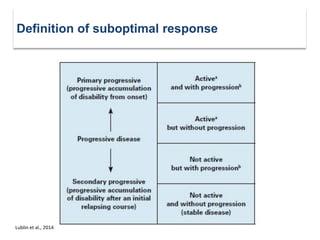
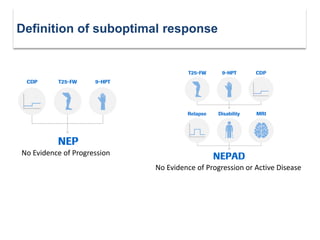
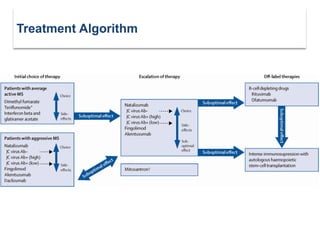
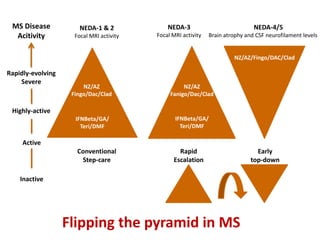
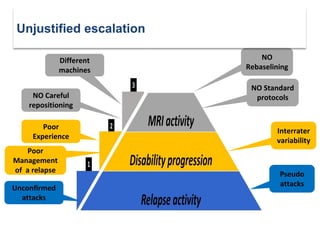
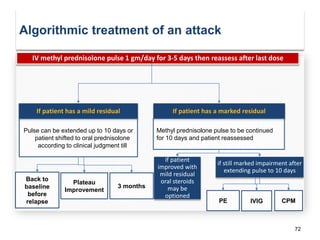
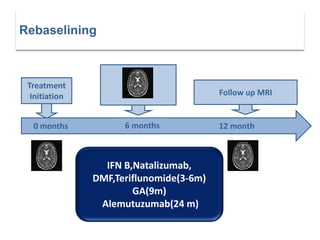
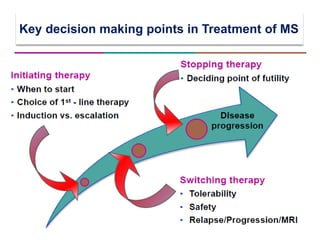
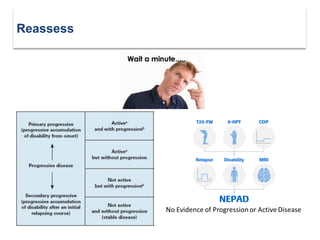
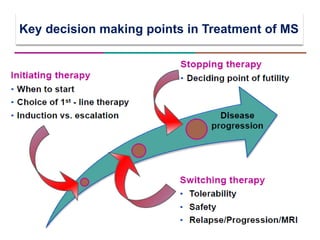
This document discusses key decision making points in the treatment of multiple sclerosis (MS). It begins with an overview of the diagnostic criteria for MS and algorithms for clinical follow up of patients with a first attack or those at risk of converting to clinically definite MS. It then covers factors to consider when choosing a first-line disease modifying therapy, including adherence, patient preferences, prognostic factors, disease activity, comorbidities, safety, tolerability, efficacy, and pregnancy plans. Specific MS comorbidities and pregnancy categories for various therapies are also summarized. The document concludes with discussions on definitions of suboptimal response, treatment algorithms, escalation versus induction strategies, and considerations for discontinuing disease modifying therapies.