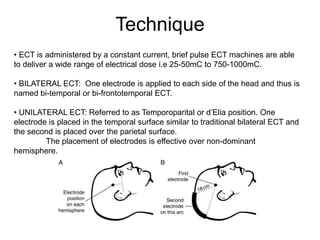
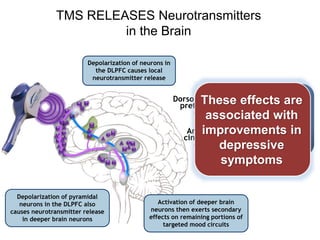
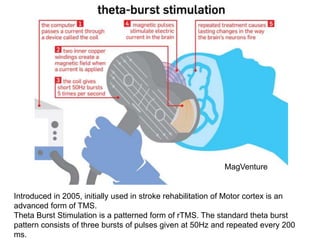
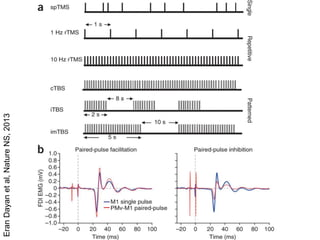
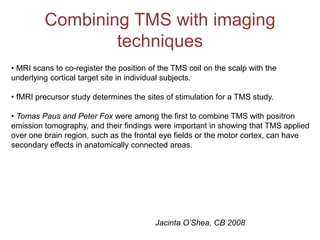
This document provides an overview of different non-invasive brain stimulation techniques, including electroconvulsive therapy (ECT), transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), and theta burst stimulation (TBS). It describes the history, mechanisms, applications, and side effects of each technique. ECT involves inducing seizures with electricity to treat conditions like depression. tDCS uses weak electrical currents to modulate cortical excitability. TMS uses electromagnetic induction to stimulate targeted brain regions without surgery. These non-invasive methods can modulate brain activity and connectivity to treat various neurological and psychiatric disorders.