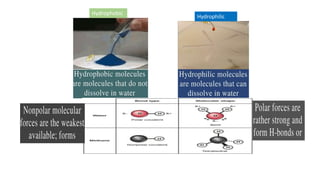
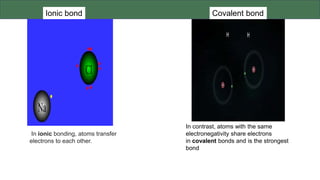
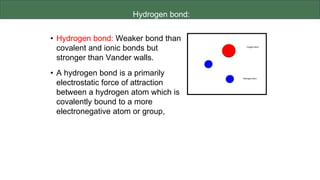
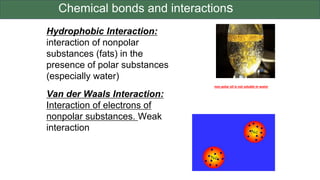
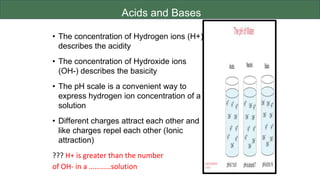
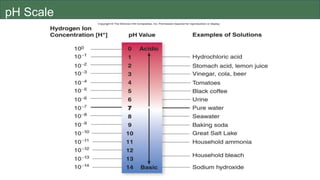
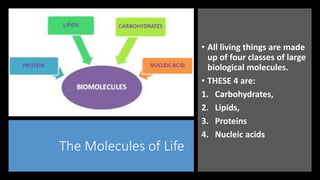
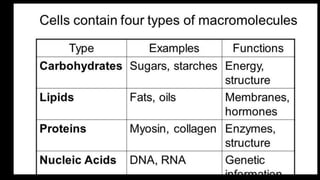
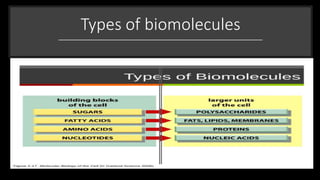
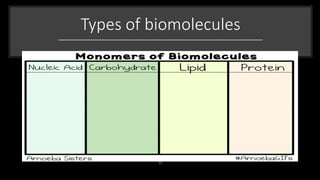
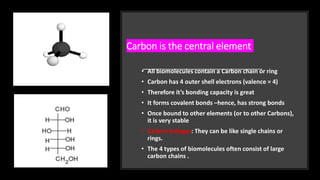
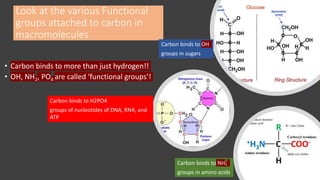
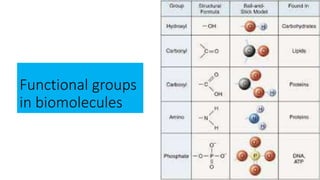
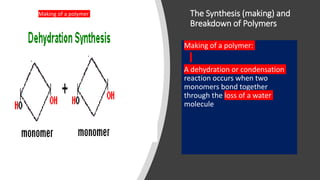
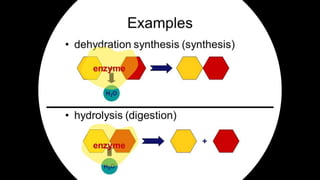
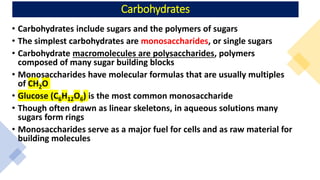
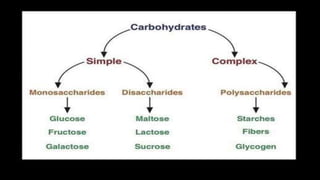
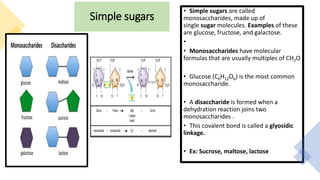
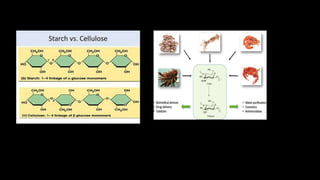
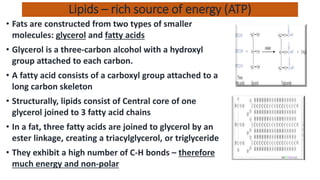
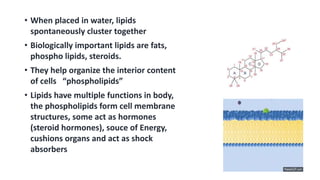
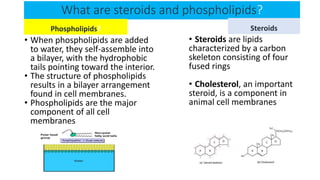
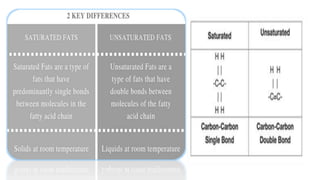
The document discusses the key components and functions of lipids. Lipids are hydrophobic molecules that are rich sources of energy. They are composed of glycerol and fatty acids. Fats are triglycerides formed from glycerol bonded to three fatty acids. Phospholipids are major components of cell membranes that form bilayers in water. Steroids have four fused carbon rings and include cholesterol, important in cell membranes. Lipids store energy, organize cell interiors, and act as hormones. Essential fatty acids must be obtained through diet.